Figure 3.
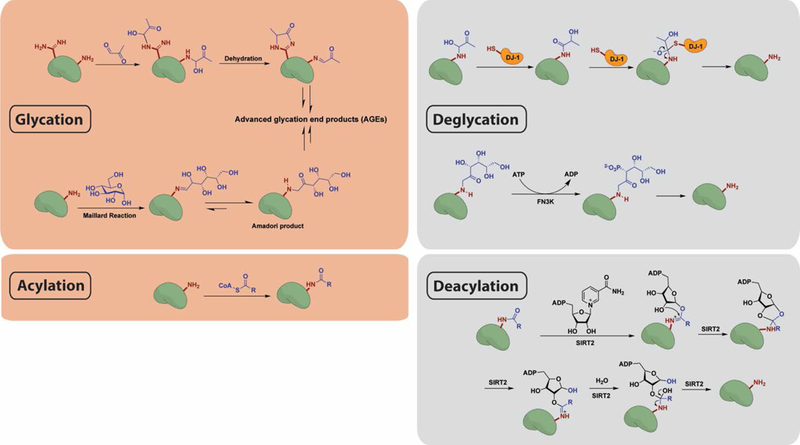
Reaction mechanisms for chemical modification and enzymatic removal of glycation and acylation marks. (Top) Lysine or arginine residues condense with aldehyde moieties in reducing sugars and can then undergo further rearrangement to yield AGEs. DJ-1 functions as a deglycase on early stage glycation adducts by participating in a nucleophilic addition–elimination reaction. FN3K phosphorylates the Amadori product of reducing sugar adducts, resulting in its destabilization and ultimate collapse. (Bottom) Lysine residues react with acyl-CoA thioesters via nucleophilic addition–elimination. By an NAD-dependent mechanism, SIRT2 removes acyl groups from the modified proteins.
