Figure 4.
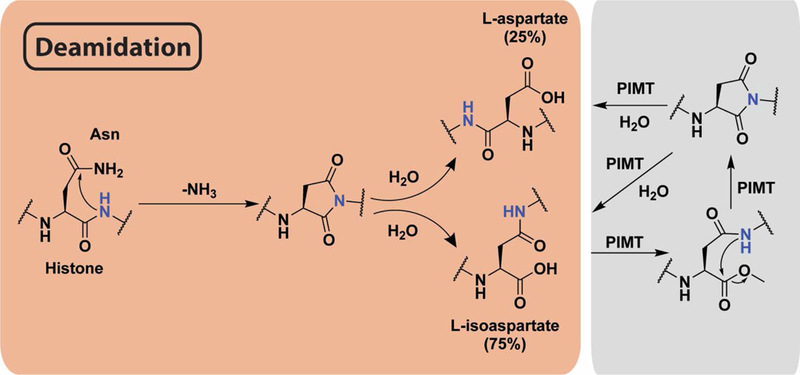
Mechanism of asparagine deamidation and its reversal. Peptide backbone nitrogen attacks the side chain amide, releasing ammonia to form a succinimide ring. Upon hydrolysis, either aspartate or isoaspartate are formed. PIMT methylates the resulting carboxylate, forming an ester that is spontaneously hydrolyzed by intramolecular attack from backbone amide nitrogen to reform the succinimide ring. Successive rounds of this mechanism may be required to fully repair deamidation.
