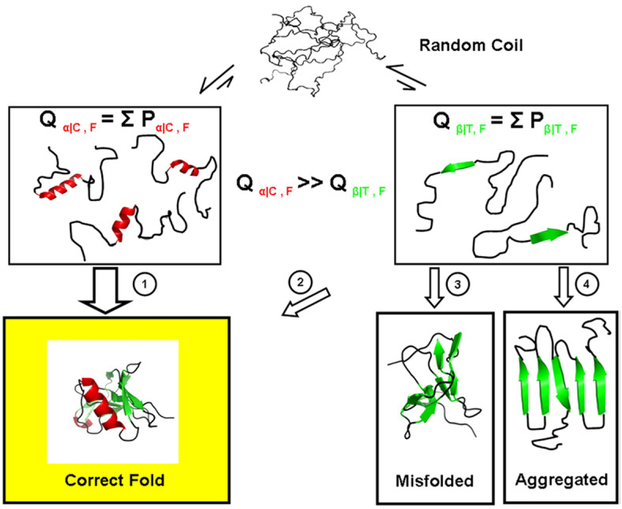
Fig. 12.
Schematic representation of the denatured-state energy landscape. Shown is a hypothetical unfolded protein (top), which is depicted as having no structural propensity. The strong negative bias for β-structure formation coupled to the modest propensity for α-helix and coil structure formation suggests that the subpartition function for states involving isolated folded segments of helix and coil (Qα/C, left) is significantly higher than the subpartition function for states where isolated segments of β-strand (Qβ/T, right) are folded. By minimizing the Qβ/T subensemble, the probability is decreased for misfolding events (pathways 3 and 4), and the folding flux25 through potentially hazardous pathways is decreased (pathway 2). We note that the precollapse equilibrium does not obligatorily signify that nucleation between different parts of the structure and subsequent folding occurs only through helix and coil, only that those segments in isolation have high folding probabilities.