Scheme 1. Synthesis of 1–3a.
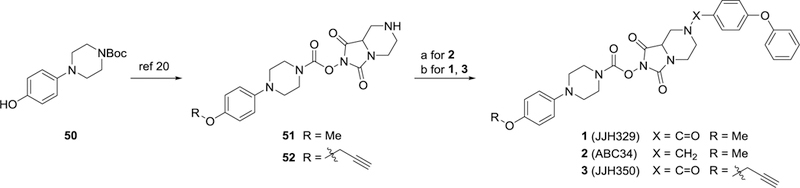
aReagents and conditions: (a) 4-phenoxybenzaldehyde, acetic acid, NaBH(OAc)3, dry THF, rt, 45% (ref 20); (b) 4-phenoxybenzoic acid, EDCI, HOBt·H2O, iPr2NEt3, rt, 74% for 2, 86% for 3.

aReagents and conditions: (a) 4-phenoxybenzaldehyde, acetic acid, NaBH(OAc)3, dry THF, rt, 45% (ref 20); (b) 4-phenoxybenzoic acid, EDCI, HOBt·H2O, iPr2NEt3, rt, 74% for 2, 86% for 3.