Scheme 3. Synthesis of 8–12a.
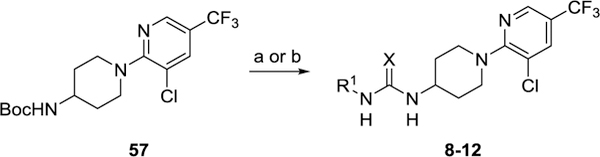
aReagents and conditions: (a) 4N HCl in 1,4-dioxane, CH2Cl2, rt then R1–NH2, phenylchlorothionoformate, iPr2NEt3, CH2Cl2, 21–45 %; (b) 4N HCl in 1,4-dioxane, CH2Cl2, rt then R1–N=C=S or R1–N=C=O, iPr2NEt3, CH2Cl2, rt, 71–100 %.
