Figure 1. Balanced redox regulation of whole cell IKv by differential incorporation of Kvβ proteins in heteromultimeric channels.
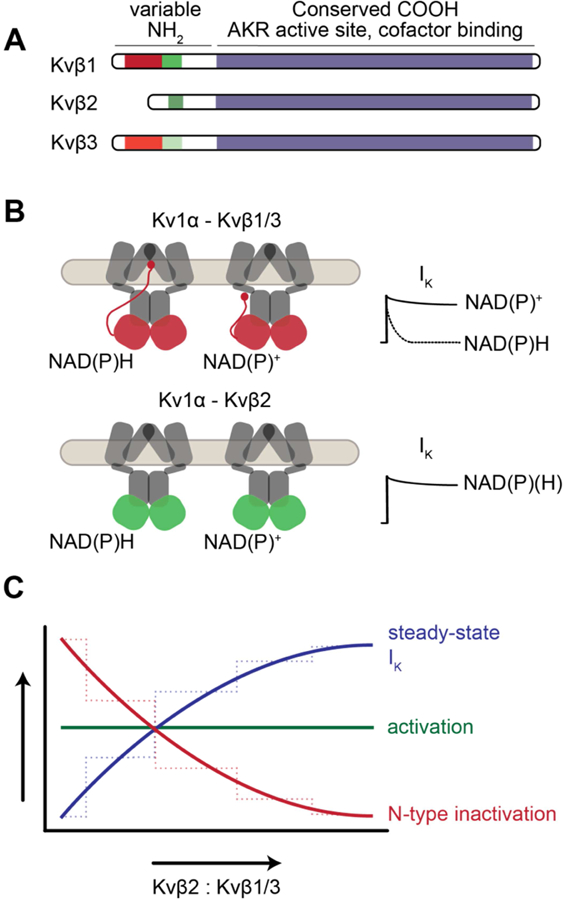
(A) Schematic of Kvβ1, Kvβ2, and Kvβ3 amino acids showing conserved COOH terminal region (blue) and variable N-termini (green/red). Several splice variants which differ in the N-terminal domain have been found for Kvβ1 (1.1–1.3_and Kvβ2 (2.1, 2.2). Ball-and-chain inactivation domain is shown in red. Adapted from [21]. (B) Differential regulation of Kv channel inactivation by Kvβ1/3 (red) and Kvβ2 (green) in the presence of oxidized and reduced pyridine nucleotides. Adapted from [54]. Kv channels expressed in membranes of excitable cells represent heterogeneous populations of structures with varying β subunit compositions. Channels that assemble with Kvβ1/Kvβ3 proteins demonstrate enhanced inactivation upon binding to reduced pyridine nucleotides. Conversely, sensitivity of channel inactivation to pyridine nucleotide redox is absent in channels that associate with Kvβ2, which does not contain the ball-and-chain. (C) Graph showing proposed regulation of Kv channel activation, inactivation, and single channel and whole-cell IKv as the ratio of expressed Kvβ2 : Kvβ1 or Kvβ3 is increased. A reduction in channel inactivation with increased Kvβ2:Kvβ1/3 in native Kv channels could produce an increase in single channel Kv activity and elevation in steady-state IK, which may lead to significant alteration in membrane excitability and responsiveness to changes in cellular metabolism. Solid lines represent predicted observed effects on whole cell Kv activation, inactivation, and IK. Dashed lines show plausible graded effects of Kvβ2:Kvβ1/3 ratio on inactivation and IK.
