Fig. 1. Geoelectrochemical metal production in the early ocean alkaline hydrothermal systems.
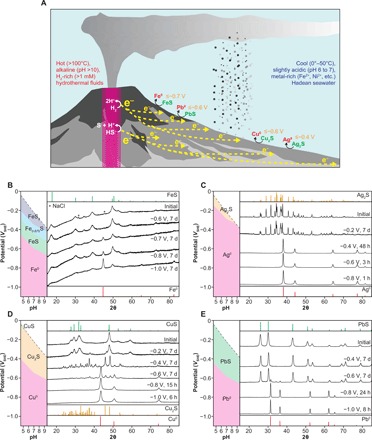
(A) At the vent-seawater interface, metal sulfides precipitated through mixing between the ancient seawater rich in metal cations (for example, Fe2+) and the alkaline hydrothermal fluid containing HS− were exposed to a negative electric potential and were electroreduced to the corresponding metals (for example, Fe0) with the reactivity depending on the potential and the nature of sulfides. (B to E) X-ray diffraction (XRD) patterns of FeS, Ag2S, CuS, and PbS before and after the electrolysis, respectively. The small peaks noted by asterisks (*) in (B) represent NaCl signals. The XRD data for the other sulfides are presented in fig. S3. The potential/pH diagrams of the relevant metal-sulfide systems are shown in the left columns. The colors represent the thermodynamically predicted stability regions of metals (red) and sulfides (green, orange, and blue refer to the sulfides with the metal/sulfur ratio of 1, >1, and <1, respectively) in the aqueous condition examined in the present study.
