Defaunation erodes millions of years of evolutionary history of bird–seed dispersal interactions in human-modified landscapes.
Abstract
Species on Earth are interconnected with each other through ecological interactions. Defaunation can erode those connections, yet we lack evolutionary predictions about the consequences of losing interactions in human-modified ecosystems. We quantified the fate of the evolutionary history of avian–seed dispersal interactions across tropical forest fragments by combining the evolutionary distinctness of the pairwise-partner species, a proxy to their unique functional features. Both large-seeded plant and large-bodied bird species showed the highest evolutionary distinctness. We estimate a loss of 3.5 to 4.7 × 104 million years of cumulative evolutionary history of interactions due to defaunation. Bird-driven local extinctions mainly erode the most evolutionarily distinct interactions. However, the persistence of less evolutionarily distinct bird species in defaunated areas exerts a phylogenetic rescue effect through seed dispersal of evolutionarily distinct plant species.
INTRODUCTION
Biotic interactions form the backbone of biological diversity while delivering the unique ecological functions essential for human and nonhuman well-being (1, 2). Their fate is defined by both the contemporary ecological correlates and the evolutionary trajectories of the interacting species (3, 4). Thus, human activities known to affect species interactions within ecological time frames may likewise affect the evolutionary history of the interaction partners. Among them, defaunation, the worldwide pervasive human-induced extinction of animal populations or entire species, substantially affects large-bodied organisms that often perform interactions whose ecological function cannot be easily replaced by smaller-sized species (5). If these species are highly evolutionarily distinct (e.g., unique lineages in the Tree of Life and those with fewer extant relatives and a longer evolutionary history), then they may harbor greater amounts of evolutionary information than expected by species number alone (6, 7). Aimed at measuring this importance, “evolutionary distinctness” (hereafter ED) estimates the contribution of a given species to the total evolutionary history of its clade while measuring its isolation in the phylogenetic tree (8, 9). We propose to expand this concept by characterizing ecological interactions according to their ED, i.e., how species with different ED values interact with each other (Fig. 1). Thus, we can assess how the contemporary fast-paced defaunation has eroded the evolutionary history embedded in these interactions. Here, we coin the term “evolutionary distinctness of the interaction” (EDi) to refer to the combined ED that both interacting partner species convey to a given interaction, irrespective of how long they have been interacting with one another.
Fig. 1. The EDi is a combination of the ED of the interacting species that can be used to characterize the evolutionary history of the seed dispersal interactions.
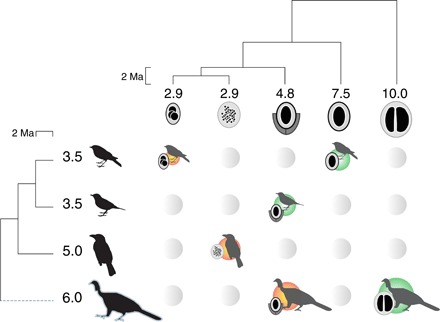
The ED of each species, calculated by equally dividing the phylogenetic distance of a branch among its daughter branches, is shown at the tips of the phylogenies. The size of the bird and plant fruit silhouettes is proportional to their ED. The ED magnitude of a bird–seed dispersal interaction ranges from very high when distinct plants are dispersed by distinct birds to very low when the opposite is true. The asymmetry can be bird-skewed, when a bird disperses a plant with lower distinctness than its own (orange circles), or plant-skewed otherwise (green circles). Defaunation is expected to have stronger impacts on interactions that involve bird species with a high ED (dashed line, highlighted bird silhouette).
Species interactions entail millions of years of reciprocal effects and a vast amount of the genetic and ecological information that characterize their unique and irreplaceable contribution to support the Earth’s biodiversity (10, 11). Mutualism rarely evolves as a process in which partners have joint, reciprocal evolutionary trajectories involving cospeciation with congruent phylogenetic branching (12, 13). Instead, species with rather different evolutionary trajectories tend to interact in contemporary habitats, showing a marked asymmetry of ED (e.g., the pollination of a basal clade angiosperm species by an insect from a recent clade). For example, the ages of nectar-feeding and fruit-eating bird and mammal families and their core plant families are consistently skewed toward older plant taxa, evidencing that most animal families are younger than their partner plant families (11). In the specific case of tropical forests, avian seed dispersal is mediated by multiple species, with generalized interactions established by the consumption of fruits that do not necessarily require specialized traits (11, 12). Yet, generalist partner species hold distinct evolutionary histories that meet in contemporary time, forming interactions that combine all the evolutionary information that the interacting actors carry.
Because large-bodied animals and large-seeded plants tend to be evolutionarily distinct species owing to both their old age and isolation in the Tree of Life (14, 15), their interactions involve high values of EDi while accounting for the largest amounts of evolutionary history. Therefore, defaunation, by reducing the populations of large-bodied bird species (5) and, consequently, the probability of interactions with large-seeded plant species, would likely extirpate the most evolutionarily distinct interactions. In contrast, lower EDi values are expected for interactions involving small-bodied bird and small-seeded plant species, which conform to be the persisting interactions in fragmented landscapes (16). Thus, both locally extinct and persisting interactions would be characterized by relatively symmetric EDi (i.e., high-ED animal–high-ED plant species for locally extinct and low-ED animal–low-ED plant species for persistent interactions). Otherwise, when the interacting partners have highly divergent ED (e.g., low-ED animal–high-ED plant species), an asymmetric EDi emerges, with unexplored outcomes on the eco-evolutionary functions that support current ecosystems (10). Therefore, defaunation, by selectively pruning species from the Tree of Life (17, 18), is causing the extirpation of interactions with distinct combinations of evolutionary history, with consequences that may go well beyond the current ecological time (19, 20).
We quantify the signature of defaunation in the ED of plant-frugivore interactions in fragmented tropical forests by combining field and phylogenetic data of the plant and bird species involved in seed dispersal. Here, we take a step forward by considering the effects of defaunation not only on species but also on the current distribution of the EDi of bird–seed dispersal interactions. We compiled 21 studies on seed dispersal by birds in which defaunation was estimated as the difference between the sum of all bird body masses at the regional landscape scale and the summed body masses of the birds that occur at the local, within-fragment scale. As shown in table S1, the studies were carried out in the Atlantic Forest of South America (21), a biodiversity hot spot (22) that has been drastically reduced to ~12% of its original cover in the past few centuries (23). The studied sites vary from small fragments of 0.66 ha to large forest remnants up to 42,000 ha along a disturbance gradient from semipristine-protected areas to secondary forests and restored plantations.
RESULTS
We found that defaunation caused the loss of interactions involving unique lineages of bird and plant species that have faced millions of years of hazards but may not be able to survive human disturbance in the Anthropocene (figs. S1 and S2). The loss of bird clades tends to concentrate on larger-bodied species, which are also the most evolutionarily distinct (r = 0.21, t = 2.78, P = 0.007; fig. S3 and table S2). These include large- and medium-bodied birds, such as guans [e.g., Pipile jacutinga (Cracidae): mean weight, 1250 g; mean ED, 28.99 million years (Ma)] and motmots [e.g., Baryphthengus ruficapillus (Momotidae): mean weight, 142 g; mean ED, 71.54 Ma] that are essential to the dispersal of large-seeded plant species with the highest ED, such as palms [e.g., Euterpe edulis (Arecaceae): mean seed diameter, 12 mm; mean ED, 98.70 Ma] and nutmegs [e.g., Virola bicuhyba (Myristicaceae): mean seed diameter, 16 mm; mean ED, 36.26 Ma] mostly found in less disturbed forests and rarely on disturbed sites. Likewise, the ED of plant species is associated with a larger seed diameter (r = 0.38, t = 5.84, P = 0.001; fig. S3 and table S3). These two effects in combination indicate that interactions involving plant and animal lineages with high ED are selectively lost in more disturbed sites (figs. S1 and S2 and table S4).
Seed dispersal interactions in the Atlantic Forest show significantly asymmetric EDi values, mostly skewed toward plant species although bird-skewed interactions are also present (Fig. 2A and table S4). Bird-plant associations present in a higher number of fragments followed the same trend (Fig. 2B), indicating that most of the persisting interactions involve less evolutionarily distinct bird species dispersing more evolutionarily distinct plants. In contrast, bird-skewed asymmetries, i.e., bird species with a higher ED that disperse plant species with lower ED, often occur in a single or very few fragments (Fig. 2B), revealing their inability to persist in defaunated landscapes.
Fig. 2. Asymmetries in the ED of seed dispersal by birds in the Atlantic Forest.
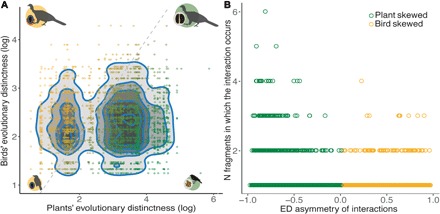
(A) A density plot showing asymmetries in ED between bird and plant species partners in seed dispersal interactions. Each dot represents a single-interaction event, and the intensity of the gray-shaded areas increases with the concentration of the most common asymmetries. Bird-skewed interactions (orange dots) occur when more evolutionarily distinct bird species disperse less evolutionarily distinct plant species; plant-skewed interactions (green dots) occur when more evolutionarily distinct plant species are dispersed by less evolutionarily distinct bird species. Plant-skewed interactions are twice the number of bird-skewed interactions (bird-skewed, 871; plant-skewed, 1797; P < 0.001, binomial test). (B) The frequency of the ED asymmetries of seed dispersal interactions in different forest fragments. Results from a generalized linear model indicate that plant-skewed asymmetries occur in a larger number of fragments (slope, − 0.09 ± 0.04, z = −2.46; P = 0.01) and thus are significantly more persistent in the fragmented landscape. The colors of the dots follow the description for plant- and bird-skewed asymmetries, as in (A).
We observed that the cumulative EDi (i.e., the total EDi per fragment) decreases with increasing defaunation (slope, −5.44 ± 2.09, t = −2. 63, P = 0.02; Fig. 3) even when fragment area (fig. S4) and interaction richness (fig. S5) are controlled for. Seed dispersal interactions vanishing in the Anthropocene represent a loss of evolutionary history that can be estimated by the cumulative EDi lost along the gradient of defaunation, i.e., 3.5 to 4.7 × 104 Ma of cumulative evolutionary time (Fig. 3). The magnitude of EDi lost in the defaunated fragments of the Atlantic Forest is similar to the marked loss of avian and bees’ evolutionary history devastated by agricultural practices worldwide (24, 25), and it is intensified in very small fragments. Furthermore, we identified that the most pristine and less defaunated forests, which are located within State Parks, harbor more cumulative EDi than expected from their interaction richness (fig. S5). On the other hand, all other fragments but two are currently supporting less EDi than expected by the local pool of species performing seed dispersal interactions. These findings show that, while the less defaunated sites within protected areas harbor interactions with irreplaceable evolutionary information and unique ecological functions, defaunation pervasively vanishes evolutionary history from forest fragments.
Fig. 3. The EDi promoted by bird-mediated seed dispersal decreases sharply along a gradient of defaunation.
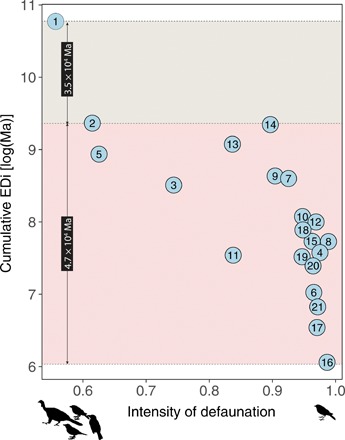
The cumulative EDi that a fragment carries corresponds to the sum of the EDi values of all interactions recorded in that fragment, here, the sum of the EDi values of all seed dispersal interactions by birds. The most pristine area is the top-left circle (1), which is the largest remnant of the Atlantic Forest and also the least defaunated fragment, used here as the baseline to illustrate the position of a “pristine” forest. The top-shaded facet of the graph represents the amount of cumulative EDi lost from the most pristine area to the second least disturbed fragment (2), while the bottom-shaded facet indicates the amount of cumulative EDi lost from the pristine area to the most disturbed fragment (16). Areas (identified by numbers within circles) are presented in a decreasing order of fragment area, according to a detailed description of each site in table S1. Larger fragments tend to be less disturbed, therefore harboring forests closer to the most pristine, best-preserved extreme.
Although overall seed dispersal interactions are drastically lost with small increases in defaunation, the different combinations of bird and plant species’ ED respond differently to this scenario (Fig. 4 and fig. S6). Interactions with bird-skewed ED values (i.e., higher ED of the avian partner, yellow lines in Fig. 4) are the first to vanish, resulting in the loss of more than 50% of interactions, with increases as small as 6% in the defaunation index. This reduction of EDi is more severe when both interacting partners are highly evolutionarily distinct (EDi from 32 to 381 Ma).
Fig. 4. Defaunation leads to an impoverishment of seed dispersal interactions and their associated asymmetries in the EDi.
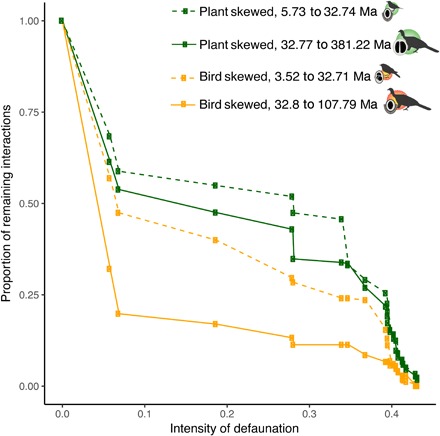
The proportion of interactions remaining in each fragment significantly decreases with defaunation, and this pattern is maintained when controlling for fragment area (fig. S5). The four types of bird–seed dispersal interaction asymmetries showed a similar trend in losing ED, but the loss was faster for bird-skewed interactions while plant-skewed interactions persisted longer in the gradient of defaunation. The 50th percentile was used as the threshold to define low and high EDi values. The defaunation index is scaled according to the lowest value among all fragments, which corresponds to the largest area and less defaunated remnant of the Atlantic Forest, used here as a baseline value to illustrate the position of a pristine forest. Continuous lines indicate high ED; dashed lines indicate low ED. The four types of asymmetry correspond to different combinations of EDi, as follows: Plant skewed, low ED (minimum EDi, 5.73 Ma; maximum EDi, 32.74 Ma); plant skewed, high ED (32.77 to 381.22 Ma); bird skewed, low ED (3.52 to 32.71 Ma); bird skewed, high ED (32.8 to 107.79 Ma).
DISCUSSION
Our findings show that the extirpated bird–seed dispersal interactions tend to include unique species that contain an irreplaceable set of genetic information that may not survive the challenges imposed by contemporary human-driven disturbances. The impoverishment of EDi in the remnants of the Atlantic Forest translates into nonrandom local extinctions of ecosystem functions related to seed dispersal by birds, such as the dispersal of large-seeded plant species. The loss of ecological functions due to reduced phylogenetic diversity in mutualistic systems has also been reported recently for agricultural landscapes in which pollination service and crop production are highly affected by the loss of highly evolutionarily distinct bee species (25). Furthermore, because the Atlantic Forest biome is mostly composed of highly defaunated small fragments (23) and because higher EDi values are restricted to a few relatively pristine and large areas, the restoration of evolutionary history would be extremely difficult with the current rates of local extinction (26). However, the reintroduction of species with higher ED can be a way forward to recover the EDi of lost interactions (27). Four of our study sites, for example, are composed of restored forests (table S1) that, similar to the highly defaunated fragments, hold low EDi values (Fig. 3), because large-seeded plant species are poorly represented in the pool of species used for restoration (28). Thus, including highly evolutionarily distinct plant species in active restoration initiatives would maximize the potential for the restoration of EDi at local and regional levels.
Overall, the loss of bird–seed dispersal interactions with plant-skewed EDi is smaller than that of bird-skewed interactions, which are the first to vanish with defaunation (Fig. 3). The fact that many plant species can be dispersed by bird species with a lower ED, which are often habitat and diet generalists (16, 29), allows them to persist longer in the defaunated areas. In this scenario, plant-skewed interactions involving small-bodied, low-ED bird species dispersing large-seeded, high-ED plant species may occur only occasionally. Thus, rare events of propagule movement are important to maintain viable populations across the fragmented landscape (30). Yet, persisting interactions with plant-skewed EDi values such as the common seed dispersal of Schinus terebinthifolia (ED, 24.5 Ma) by Turdus rufiventris (ED, 6.2 Ma) or of Trema micrantha (ED, 41.2 Ma) by Dacnis cayana (ED, 7.1 Ma) may be more stable in the environmental changes seen in the Anthropocene (31).
Our results suggest that the loss of evolutionary history of plant species in the Atlantic Forest is minimized by the rescuing effect performed by bird species with limited ED dispersing unique plant species. The rescuer bird species represent clades of small-bodied, generalist bird species able to thrive in human-modified environments, which functionally “help” some plant species to persist. The functional role of rescuer species includes the dispersal of plant species whose primary seed dispersers have been already lost (20) or functionally extinct because of overhunting, for instance (32). The large seeds of a keystone, highly evolutionarily distinct tropical palm, E. edulis, have rapidly evolved toward smaller sizes in populations in which its main large-bodied, evolutionarily distinct seed disperser, P. jacutinga, has been locally extinct (33). The response of E. edulis to defaunation may increase its probability of being phylogenetically rescued by less distinct, small-bodied bird species.
Yet, rescue effects cannot functionally replace lost interactions. The phenotypic and microevolutionary changes observed in plants that lost their primary large-bodied seed dispersers (33, 34) also indicate that, although small birds can still disperse the smaller seeds, they do not functionally replace the effectiveness of the seed dispersal service provided by the vanished large-bodied frugivores. Therefore, the rescue effect cannot recover the extinction of the unique evolutionary histories of the interactions affected by defaunation. Moreover, the predominance of plant-skewed asymmetries in the Atlantic Forest may be a reflection of the extinction debt of interactions [sensu (19)]. Such a debt means that plants, which have longer life spans than birds, may persist for decades after the interaction has disappeared.
Given the scenario of increasing defaunation in human-altered fragmented landscapes, a marked reshaping of the Tree of Life may be occurring in the Anthropocene. Large-bodied frugivores have been hypothesized to reduce speciation rates of large-fruited plants as a consequence of high gene flow associated with long-distance dispersal ranges, while small-fruited plants tend to speciate more owing to restricted dispersal by small-bodied frugivores (35). Therefore, the phylogenetic rescue effect promoted by the persistence of plant-skewed, low-ED interactions would favor higher rates of speciation in small-seeded plant species, which could affect the functioning of tropical forests. Furthermore, local assemblages facing larger losses of EDi can be expected to be less resilient to disturbance owing to the impoverishment of the functional diversity concomitantly occurring with the loss of phylogenetic history of the partner species (10, 36). This impoverishment is likely to change the niche space occupied by the local assemblage of interacting species (37). New empty niches may create opportunities for the establishment of exotic species (38) and trigger changes in biogeochemical cycles such as CO2 storage by large-seeded plants dispersed by large-bodied frugivores (39). The resultant Anthropocene landscape will most likely consist of a metacommunity of evolutionarily impoverished fragments, phylogenetically structured by the defaunation of specific clades (40). Our approach can be easily extended to other ecological systems from which similar results are expected on the basis of the loss of specific functional groups, such as the global decline of bee pollinators (6, 25). From a conservation point of view, our results show that we are losing unique evolutionary histories of interactions that cannot be replaced by contemporaneous species. However, these losses may be lessened by a few persisting interactions performed by less evolutionarily distinct bird species rescuing the more evolutionarily distinct plant species from the verge of extinction.
MATERIALS AND METHODS
Study area
The Atlantic Forest is a tropical hot spot of biodiversity (22), highly threatened by human activities (such as logging, habitat conversion to commercial monocultures, and increasing urbanization), that has drastically reduced its original cover to ~12% (23). The remaining landscape constitutes a complex mosaic formed mainly by small fragments (<50 ha, ~80% of the remaining area) and clusters of close neighboring fragments (<200 m apart) (23). These fragments function as a metanetwork of interactions connected by small generalist bird species dispersing small-seeded plant species, while large-bodied bird species able to disperse large-seeded plant species are constrained to a few pristine, large area fragments (16).
Dataset
We used the Atlantic-Frugivory dataset, the largest dataset available for tropical plant-frugivore interactions (21), to test how much evolutionary history of bird–seed dispersal interactions is being lost because of the contemporaneous defaunation. Bird and plant species present in the Atlantic-Frugivory dataset comprise our regional pool of species, which was used to build the correspondent phylogenies and to calculate the defaunation index (see details in the next subsections). From that regional pool, we selected 21 studies of bird–seed dispersal interactions sampled in fragments of the Brazilian Atlantic Forest (table S1). The studied fragments vary from 0.66 to 42,000 ha in a gradient of disturbance from semipristine-protected areas to secondary forests, degraded fragments and restored plantations. The studied fragments were distributed among different vegetation types of the biome, including Ombrophilous and Semidecidous forest, Araucaria Forest, rupestrian, and dry vegetation. Our dataset includes all studies with available information designed to collect fruit-eating bird interactions at the community level in the Atlantic Forest (21); yet, they did not necessarily record effective seed dispersal. We carefully checked the dataset and removed any interaction that would not characterize seed dispersal events. Methods to record interactions varied among studies, including direct bird observations and fecal sampling; only community-level studies with at least 12 months of sampling were included in the analyses. We further added available information to each study site based on field observations and expert knowledge about tropical avian seed dispersal.
Phylogenetic trees
Birds
Given the list of species at the regional pool (21), we obtained 100 phylogenetic trees inferred from the BirdTree.org online database using the “Stage2 MayrParSho Ericson” source tree as birds’ master phylogeny (available at http://birdtree.org/methods/). Trees’ topology is resolved with a combination of genetic and taxonomic information and dated with a relaxed molecular clock [see (6, 41) for details]. A few species that were identified at genus level in the field (i.e., Elaenia sp., Myiarchus sp., Patagioenas sp., Penelope sp., and Turdus sp.) therefore could not be found in the master phylogeny. We randomly grafted these species in their correspondent clades using the “add.species.to.genus” function in the phytools package version 0.6-00 in R (42).
Plants
Given the list of plant species at the regional pool (21), we used the megaphylogeny of trees (43) with the “S.PhyloMaker” function (41) to obtain the initial plant tree. The initial tree was based on “scenario 1” in which missing genera and/or species are added as basal polytomies within their higher taxonomic level (44). Then, we simultaneously resolved the polytomies and adjusted the branch lengths using an evolutionary birth-death model with the help of “PolytomyResolver” function (45) and BEAST version 1.5.4 (46). Markov Chain Monte Carlo analyses were run for 106 iterations, and trees were sampled every 103 iterations. After discarding the 25% first trees, we randomly selected 100 trees for subsequent analyses.
ED of bird–seed dispersal interactions
The ED of a given species quantifies how isolated it is in its own phylogenetic tree and the amount of unique evolutionary history that it embodies (8, 9). Similarly, the amount of evolutionary history that an interaction holds can be estimated by the combination of the ED of the interacting partner species. Therefore, the EDi combines the isolation and unique evolutionary history of the partner species in their correspondent phylogenetic trees. We calculated EDi as follows.
First, we calculated the ED for all plant and bird species based on the correspondent phylogenetic trees that we built for the regional pool of species of the Atlantic Forest (see above). ED was estimated using the equal splits metric available in the “evol.distinct” function in the picante package for R (47). Equal splits metric equally divides the phylogenetic distance represented by a branch by the number of lineages originating from the node directly below it (8). It gives higher values of ED for species placed in clades in which the path length is shared among a lower number of species and is considered a robust metric to capture phylogenetic diversity (9). Then, we obtained the mean ED for each bird (EDb) and plant species (EDp) calculated over 100 solved trees and used it as a proxy for its ED. Last, to quantify the EDi, we summed the ED of the interacting bird (EDb) and plant species (EDp) such as EDi = EDb + EDp.
Statistical analyses
To test whether more evolutionarily distinct bird and plant species were associated with larger body mass and seed diameter, respectively, we used nonparametric Spearman correlation tests (48). The extremely large sample size caused an overfit of any modeling attempt to assess the form of these relationships, so we resorted to use nonparametric Spearman correlation tests to highlight any nonrandom associations between traits and ED.
Asymmetries in EDi were calculated as the difference between the ED values of both interacting partners. Bird-skewed asymmetries correspond to interactions in which the bird species has higher ED than the partner plant species, and plant-skewed asymmetries correspond to interactions in which the plant species brings more ED than the partner bird species. We apply a binomial test to estimate which type of asymmetry (bird skewed or plant skewed) is significantly more predominant among the 2668 unique combinations of bird and plant species interactions found in our communities. Then, we used a generalized linear mixed-effects model in the lme4 package (49) to test which type of EDi asymmetry is more persistent in the fragmented landscape. In this model, we fit the number of fragments in which the interaction occurs as a response variable against the asymmetry value of EDi as the independent variable. Bird species and plant species were used as random factors to control for pseudoreplication, given that a single species can perform more than one interaction. We fit the model with a Poisson distribution.
To understand the amount of the cumulative EDi of bird–seed dispersal interactions lost in the gradient of defaunation across tropical forest fragments, we fitted three different linear models. We started by quantifying the cumulative EDi in each fragment by summing up the EDi values of all bird–seed dispersal interactions recorded per fragment. Defaunation was estimated as the difference between the sum of all bird body masses at the regional landscape scale [considering all frugivore bird species present in the Atlantic-Frugivory database (21) as a proxy for the regional pool of species] and the summed body masses of the birds that occur in each fragment. For the first model, we simply used the cumulative EDi as the response variable fitted against the defaunation index to test for the absolute loss of EDi as defaunation increases. For the second model, we used the same approach while controlling for fragment area. We tested for the correlation between fragment area and defaunation using a Pearson correlation test. Then, we used the residuals of a linear regression of defaunation index on fragment area as the independent variable fitted against the cumulative EDi. Last, we used a null model to test whether the cumulative EDi per fragment was significantly different from that expected by the number of bird–seed dispersal interactions recorded in each community. We created an amn matrix in which m and n correspond to the study site and the bird–seed dispersal interactions, respectively. The amn element of the matrix is filled according to the presence of an interaction n in site m quantified by the corresponding EDi. Therefore, the marginal total of m corresponds to its observed cumulative EDi. We applied the “shuffle.web” method within the “nullmodel” function in the bipartite package (50), which relocates the amn element within the matrix while maintaining its dimensionality, i.e., constraining the number of sites and interactions while shuffling the EDi among them. We obtained the mean and SD of the EDi per site over 100 simulations and calculated the corresponding z score based on the observed cumulative EDi. The z scores were fitted against the defaunation index in a linear model. Positive z scores indicate that the site holds more cumulative EDi than expected by the number of bird-plant interactions recorded in that area. On the other hand, negative z scores indicate that the site holds less cumulative EDi than expected by the number of interactions that it encompasses. Statistical analyses were performed in R version 3.4.3 (51).
Supplementary Material
Acknowledgments
We thank V. B. Zipparro for plant taxonomy updates and S. Timotéo, M. A. Mello, and N. Villar for invaluable suggestions to an early draft of this manuscript. Funding: Our study was supported by the São Paulo Research Foundation (FAPESP: 2014/01986-0, 2015/15172-7, 2016/18355-8, and 2017/25605-3). M.A.P. and M.G. received fellowships from the Brazilian Council of Scientific and Technological Development (CNPq). P.J. was supported by the Severo Ochoa Excellence Award (SEV-2012-0262), grant CGL2017-82847P, and the Science Without Borders Program (CNPq, PVE-401258/2012). M.V. was supported by the Ecometas excellence network (CGL2016-81706-REDT). Author contributions: C.E., M.G., P.J., and M.V. designed the study. M.G., P.J., and M.A.P. collected part of the data. C.E. wrote the first draft of the manuscript. All authors contributed to the discussion and reviewed the final text. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Raw data are available at https://github.com/LEEClab/Atlantic_series and at https://github.com/carineemer/Phylogenies_Seed_Dispersal.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/6/eaav6699/DC1
Fig. S1. Distribution of the ED of the frugivore bird species in the gradient of defaunation across the fragments of the Atlantic Forest.
Fig. S2. Distribution of the ED of the bird-dispersed plant species in the gradient of defaunation across the fragments of the Atlantic Forest.
Fig. S3. Correlation between the ED and the traits of the species involved in seed dispersal interactions in the Atlantic Forest.
Fig. S4. Defaunation leads to a significant impoverishment of the cumulative EDi performed by bird seed dispersal across forest fragments.
Fig. S5. EDi promoted by bird seed dispersal decreases sharply along a gradient of defaunation.
Fig. S6. Defaunation leads to an impoverishment of seed dispersal interactions and their associated asymmetric relationships of EDi.
Table S1. Description of the dataset used in this study.
Table S2. Traits of the bird species recorded in the studied communities of the Atlantic Forest.
Table S3. Traits of the plant species recorded in the studied communities of the Atlantic Forest.
Table S4. The bird–seed dispersal interactions recorded in the studied sites of the Atlantic Forest, followed by their EDi and correspondent asymmetry.
REFERENCES AND NOTES
- 1.Mace G. M., Gittleman J. L., Purvis A., Preserving the tree of life. Science 300, 1707–1709 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Cardinale B. J., Duffy J. E., Gonzalez A., Hooper D. U., Perrings C., Venail P., Narwani A., Mace G. M., Tilman D., Wardle D. A., Kinzig A. P., Daily G. C., Loreau M., Grace J. B., Larigauderie A., Srivastava D. S., Naeem S., Biodiversity loss and its impact on humanity. Nature 486, 59–67 (2012). [DOI] [PubMed] [Google Scholar]
- 3.G. E. Hutchinson, The Ecological Theatre and the Evolutionary Play (Yale Univ. Press, 1965). [Google Scholar]
- 4.C. C. Labandeira, The history of associations between plants and animals, in Plant-Animal Interactions: An Evolutionary Approach, C. M. Herrera, O. Pellmyr, Eds. (Blackwell Scientific, 2002). [Google Scholar]
- 5.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B., Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Jetz W., Thomas G. H., Joy J. B., Redding D. W., Hartmann K., Mooers A. O., Global distribution and conservation of evolutionary distinctness in birds. Curr. Biol. 24, 919–930 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Sechrest W., Brooks T. M., da Fonseca G. A. B., Konstant W. R., Mittermeier R. A., Purvis A., Gittleman J. L., Hotspots and the conservation of evolutionary history. Proc. Natl. Acad. Sci. U.S.A. 99, 2067–2071 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redding D. W., Mooers A. Ø., Incorporating evolutionary measures into conservation prioritization. Conserv. Biol. 20, 1670–1678 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Redding D. W., Hartmann K., Mimoto A., Bokal D., DeVos M., Mooers A. Ø., Evolutionarily distinctive species often capture more phylogenetic diversity than expected. J. Theor. Biol. 251, 606–615 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Srivastava D. S., Cadotte M. W., MacDonald A. M., Marushia R. G., Mirotchnick N., Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648 (2012). [DOI] [PubMed] [Google Scholar]
- 11.T. H. Fleming, W. J. Kress, The Ornaments of Life: Co Evolution and Conservation in the Tropics (University of Chicago Press, 2013). [Google Scholar]
- 12.Guimarães P. R., Jordano P., Thompson J. N., Evolution and coevolution in mutualistic networks. Ecol. Lett. 14, 877–885 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Ponisio L. C., M’Gonigle L. K., Coevolution leaves a weak signal on ecological networks. Ecosphere 8, e01798 (2017). [Google Scholar]
- 14.Isaac N. J. B., Turvery S. T., Collen B., Waterman C., Baillie E. M., Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLOS ONE 2, e296 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igea J., Miller E. F., Papadopulos A. S. T., Tanentzap A. J., Seed size and its rate of evolution correlate with species diversification across angiosperms. PLOS Biol. 15, e2002792 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emer C., Galetti M., Pizo M. A., Guimarães P. R. Jr., Piratelli A., Moraes S., Jordano P., Seed-dispersal interactions in fragmented landscapes: A meta-network approach. Ecol. Lett. 21, 484–493 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Purvis A., Agapow P.-M., Gittleman J. L., Mace G. M., Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Rezende E. L., Lavabre J. E., Guimarães P. R., Jordano P., Bascompte J., Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448, 925–928 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Valiente-Banuet A., Aizen M. A., Alcántara J. M., Arroyo J., Cocucci A., Galetti M., Garcia M. B., Garcia D., Gómez J. M., Jordano P., Medel R., Navarro L., Obeso J. R., Oviedo R., Ramírez N., Rey P. J., Traveset A., Verdú M., Zamora R., Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307 (2014). [Google Scholar]
- 20.Galetti M., Moleón M., Jordano P., Pires M. M., Guimarães P. R., Pape T., Nichols E., Hansen D., Olesen J. M., Munk M., de Mattos J. S., Schweiger A. H., Owen-Smith N., Johnson C. N., Marquis R. J., Svenning J.-C., Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 93, 845–862 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Bello C., Galetti M., Montan D., Pizo M. A., Mariguela T. C., Culot L., Bufalo F., Labecca F., Pedrosa F., Constantini R., Emer C., Silva W. R., da Silva F. R., Ovaskainen O., Jordano P., Atlantic frugivory: A plant-frugivore interaction data set for the Atlantic Forest. Ecology 98, 1729 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J., Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 24.Frishkoff L. O., Karp D. S., M’Gonigle L. K., Mendenhall C. D., Zook J., Kremen C., Hadly E. A., Daily G. C., Loss of avian phylogenetic diversity in neotropical agricultural systems. Science 345, 1343–1346 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Grab H., Branstetter M. G., Amon N., Urban-Mead K. R., Park M. G., Gibbs J., Blitzer E. J., Poveda K., Loeb G., Danforth B. N., Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363, 282–284 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Canale R., Peres C. A., Guidorizzi C. E., Gatto C. A. F., Kierulff M. C., Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLOS ONE 7, e41671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thévenin C., Mouchet M., Robert A., Kerbiriou C., Sarrazin F., Reintroductions of birds and mammals involve evolutionarily distinct species at the regional scale. Proc. Natl. Acad. Sci. U.S.A. 115, 3404–3409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brancalion P. H. S., Bello C., Chazdon R. L., Galetti M., Jordano P., Lima R. A. F., Medina A., Pizo M. A., Reid J. L., Maximizing biodiversity conservation and carbon stocking in restored tropical forests. Conserv. Lett. 11, e12454 (2018). [Google Scholar]
- 29.M. A. Pizo, Frugivory by birds in degraded areas of Brazil in Seed-Dispersal: Theory and Its Application in a Changing World, A. J. Dennis, E. W. Schupp, R. J. Green, D. W. Westcott, Eds. (CABI International, Wallingford, UK, 2007), pp. 615–627. [Google Scholar]
- 30.García C., Borda-de-Água L., Extended dispersal kernels in a changing world: Insights from statistics of extremes. J. Ecol. 105, 63–74 (2017). [Google Scholar]
- 31.Toju H., Yamamichi M., Guimarães P. R., Olesen J. M., Mougi A., Yoshida T., Thompson J. N., Species-rich networks and eco-evolutionary synthesis at the metacommunity level. Nat. Ecol. Evol. 1, 0024 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Young H. S., McCauley D. J., Galetti M., Dirzo R., Patterns, causes and consequences of Anthropocene defaunation. Annu. Rev. Ecol. Evol. Syst. 47, 333–358 (2016). [Google Scholar]
- 33.Galetti M., Guevara R., Côrtes M. C., Fadini R., Matter S. V., Leite A. B., Labecca F., Ribeiro T., Carvalho C. S., Collevatti R. G., Pires M. M., Guimarães P. R., Brancalion P. H., Ribeiro M. C., Jordano P., Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Carvalho C. S., Galetti M., Colevatti R. G., Jordano P., Defaunation leads to microevolutionary changes in a tropical palm. Sci. Rep. 6, 31957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onstein R. E., Baker W. J., Couvreur T. L. P., Faurby S., Svenning J.-C., Kissling W. D., Frugivory-related traits promote speciation of tropical palms. Nat. Ecol. Evol. 1, 1903–1911 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Cadotte M. W., Dinnage R., Tilman D., Phylogenetic diversity promotes ecosystem stability. Ecology 93, S223–S233 (2012). [Google Scholar]
- 37.Vitória R. S., Vizentin-Bugoni J., Duarte L. D. S., Evolutionary history as a driver of ecological networks: A case study of plant-hummingbird interactions. Oikos 127, 561–569 (2018). [Google Scholar]
- 38.Traveset A., Richardson D. M., Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 45, 89–113 (2014). [Google Scholar]
- 39.Bello C., Galetti M., Pizo M. A., Magnago L. F. S., Rocha M. F., Lima R. A. F., Peres C. A., Ovaskainen O., Jordano P., Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aizen M. A., Gleiser G., Sabatino M., Gilarranz L. J., Bascompte J., Verdú M., The phylogenetic structure of plant-pollinator networks increases with habitat size and isolation. Ecol. Lett. 29, 29–36 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. Ø., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 43.Zanne A. E., Tank D. C., Cornwell W. K., Eastman J. M., Smith S. A., FitzJohn R. G., McGlinn D. J., O’Meara B. C., Moles A. T., Reich P. B., Royer D. L., Soltis D. E., Stevens P. F., Westoby M., Wright I. J., Aarssen L., Bertin R. I., Calaminus A., Govaerts R., Hemmings F., Leishman M. R., Oleksyn J., Soltis P. S., Swenson N. G., Warman L., Beaulieu J. M., Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Qian H., Jin Y., An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 9, 233–239 (2016). [Google Scholar]
- 45.Kuhn T. S., Mooers A. Ø., Thomas G. H., A simple polytomy resolver for dated phylogenies. Methods Ecol. Evol. 2, 427–436 (2011). [Google Scholar]
- 46.Drummond A. J., Suchard M. A., Xie D., Rambaut A., Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kembel S. W., Cowan P. D., Helmus M. R., Cornwell W. K., Morlon H., Ackerly D. D., Blomberg S. P., Webb C. O., Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 48.P. Legendre, L. Legendre, Numerical Ecology (Elsevier Science, ed. 2, 1998). [Google Scholar]
- 49.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 50.Dormann C. F., Jochen F., Blüthgen N., Gruber B., Indices, graphs and null models: Analyzing bipartite ecological networks. The Open Ecol. J. 2, 7–24 (2009). [Google Scholar]
- 51.R Development Core Team, R: A language and environment for statistical computing. (2014).
- 52.W. R. Silva, P. de Marco, E. Hasui, V. S. M. Gomes. Patterns of fruit-frugivores interactions in two Atlantic Forest bird communities of South-eastern Brazil: Implications for conservation, in Seed Dispersal and Frugivory: Ecology, Evolution and Conservation, D. J. Levey, W. R. Silva, M. Galetti, Eds. (CABI International, 2002), pp. 423–435. [Google Scholar]
- 53.S. B. M. Rodrigues, Rede de interações entre aves frugívoras e plantas em uma área de Mata Atlântica no sudeste do Brasil, Master thesis, UFSCAR, Sorocaba, BR (2015). [Google Scholar]
- 54.Parrini R., Pacheco J. F., Mallet-Rodrigues F., Frugivoria em Tangara desmaresti (Passeriformes: Thraupidae) na Floresta Atlântica do Parque Nacional da Serra dos Órgãos e adjacências, Estado do Rio de Janeiro, sudeste do Brasil. Atualidades Ornitológicas 142, 10–13 (2008). [Google Scholar]
- 55.Parrini R., Pacheco J. F., Haefeli L., Observação de aves se alimentando dos frutos de Miconia sellowiana (Melastomataceae) na Floresta Atlântica Alto-Montana do Parque Nacional da Serra dos Órgãos e do Parque Nacional do Itatiaia, região sudeste do Brasil. Atualidades Ornitológicas 146, 4–7 (2009). [Google Scholar]
- 56.Parrini R., Pacheco J. F., Frugivoria por aves em seis espécies arbóreas do gênero Miconia (Melastomataceae) na Mata Atlântica do Parque Nacional da Serra dos Órgãos, Região Sudeste do Brasil. Atualidades Ornitológicas 159, 51–58 (2011a). [Google Scholar]
- 57.Parrini R., Pacheco J. F., Frugivoria por aves em Alchornea triplinervia (Euphorbiaceae) na Mata Atlântica do Parque Estadual dos Três Picos, Estado do Rio de Janeiro, Brasil. Atualidades Ornitológicas 162, 33–41 (2011). [Google Scholar]
- 58.Parrini R., Pacheco J. F., Aspectos da frugivoria por aves em Cupania oblongifolia (Sapindaceae) na Mata Atlântica do Parque Nacional da Serra dos Órgãos, Estado do Rio de Janeiro, Brasil. Atualidades Ornitológicas 178, 55–62 (2014). [Google Scholar]
- 59.R. Parrini, Quatro estações, história natural das aves na Mata Atlântica: Uma abordagem trófica (Technical Books, 2015). [Google Scholar]
- 60.Parrini R., Pardo C. S., Pacheco J. F., Conhecendo as plantas cujos frutos e recursos florais são consumidos pelas aves na Mata Atlântica do Parque Nacional da Serra dos Órgãos. Atualidades Ornitológicas 199, 38–136 (2017). [Google Scholar]
- 61.V. S.M. Gomes, Variação espacial e dieta de aves terrestres na Restinga de Jurubatiba, RJ. PhD Thesis, UFRJ, Rio de Janeiro, BR (2006). [Google Scholar]
- 62.E. R. Castro, Fenologia reprodutiva do palmito Euterpe edulis (Arecaceae) e sua influência na abundância de aves frugívoras na Floresta Atlântica. PhD Thesis, UNESP Rio Claro, BR (2015). [Google Scholar]
- 63.Scherer A., Maraschin-Silva F., Baptista L. R. M., Padrões de interações mutualísticas entre espécies arbóreas e aves frugívoras em uma comunidade de Restinga no Parque Estadual de Itapuã, RS, Brasil. Acta Bot. Bras. 21, 203–212 (2007). [Google Scholar]
- 64.J. M. S. Correia. Utilização de espécies frutíferas da Mata Atlântica na alimentação da avifauna da Reserva Biológica de Poços das Antas, RJ. MSc Dissertation, UNB Brasília, BR (1997).
- 65.Manhães M. A., Dieta de Traupíneos (Passeriformes, Emberizidae) no Parque Estadual do Ibitipoca, Minas Gerais, Brasil. Iheringia 93, 59–73 (2003). [Google Scholar]
- 66.M. M. A. de Oliveira. Frugivoria por aves em um fragmento de floresta de restinga no Estado do Espírito Santo, Brasil, PhD Thesis, UNICAMP, Campinas, BR (1999). [Google Scholar]
- 67.K. J. F. Alves. Composição da avifauna e frugivoria por aves em um mosaico sucessional na Mata Atlântica. MSc Dissertation, UNESP Rio Claro, BR (2008).
- 68.Fadini R. F., de Marco P. Jr., Interações entre aves frugívoras e plantas em um fragmento de Mata Atlântica de Minas Gerais. Ararajuba 12, 97–103 (2004). [Google Scholar]
- 69.A. Kindel. Interações entre plantas ornitocóricas e aves frugívoras na Estação Ecológica de Aracuri, Muitos Capões, RS. MSc Dissertation, UFRGS Porto Alegre, BR (1996).
- 70.Galetti M., Pizo M. A., Fruit eating birds in a forest fragment in southeastern Brazil. Ararajuba 4, 71–79 (1996). [Google Scholar]
- 71.Pizo M. A., Frugivory and habitat use by fruit-eating birds in a fragmented landscape of southeast Brazil. Ornitol. Neotrop. 15, 117–126 (2004). [Google Scholar]
- 72.S. Athiê. Composição da avifauna e frugivoria por aves em um mosaico de vegetação secundária em Rio Claro, região centro-leste do estado de São Paulo, MSc Dissertation, UFSCar São Carlos, BR (2009).
- 73.da Silva F. R., Montoya D., Furtado R., Memmott J., Pizo M. A., Rodrigues R. R., The restoration of tropical seed dispersal networks. Restor. Ecol. 23, 852–860 (2015). [Google Scholar]
- 74.E. Hasui, O papel das aves frugívoras na dispersão de sementes em um fragmento de floresta semidecídua secundária em São Paulo, SP. MSc Dissertation, USP São Paulo, BR (1994).
- 75.V. Robinson, Índice de importância das aves como dispersoras de sementes para uma comunidade vegetal reflorestada em Piracicaba, BSc Dissertation, UNESP, Rio Claro, BR (2015).
- 76.R. F. de M. Silva, Interações entre plantas e aves frugívoras no campus da Universidade Federal do Rio de Janeiro, BSc Dissertation, UFRJ Rio de Janeiro, BR (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/6/eaav6699/DC1
Fig. S1. Distribution of the ED of the frugivore bird species in the gradient of defaunation across the fragments of the Atlantic Forest.
Fig. S2. Distribution of the ED of the bird-dispersed plant species in the gradient of defaunation across the fragments of the Atlantic Forest.
Fig. S3. Correlation between the ED and the traits of the species involved in seed dispersal interactions in the Atlantic Forest.
Fig. S4. Defaunation leads to a significant impoverishment of the cumulative EDi performed by bird seed dispersal across forest fragments.
Fig. S5. EDi promoted by bird seed dispersal decreases sharply along a gradient of defaunation.
Fig. S6. Defaunation leads to an impoverishment of seed dispersal interactions and their associated asymmetric relationships of EDi.
Table S1. Description of the dataset used in this study.
Table S2. Traits of the bird species recorded in the studied communities of the Atlantic Forest.
Table S3. Traits of the plant species recorded in the studied communities of the Atlantic Forest.
Table S4. The bird–seed dispersal interactions recorded in the studied sites of the Atlantic Forest, followed by their EDi and correspondent asymmetry.


