Abstract
Purpose
To determine the diagnostic value of eosinopenia and the neutrophil-to-lymphocyte ratio (NLR) in the diagnosis of early onset neonatal sepsis (EONS).
Methods
This cross-sectional study was conducted in the Neonatology Ward of R.D. Kandou General Hospital Manado between July and October 2017. Samples were obtained from all neonates meeting the inclusion criteria for EONS. Data were encoded using logistic regression analysis, the point-biserial correlation coefficient, chi-square test, and receiver operating characteristic curve analysis, with a P value <0.05 considered significant.
Results
Of 120 neonates who met the inclusion criteria, 73 (60.8%) were males and 47 (39.2%) were females. Ninety (75%) were included in the sepsis group and 30 (25%) in the nonsepsis group. The mean eosinophil count in EONS and non-EONS groups was 169.8±197.1 cells/mm3 and 405.7±288.9 cells/mm3 , respectively, with statistically significant difference (P<0.001). The diagnostic value of eosinopenia in the EONS group (cutoff point: 140 cells/mm3 ) showed 60.0% sensitivity and 90.0% specificity. The mean NLR in EONS and non-EONS groups was 2.82±2.29 and 0.82±0.32, respectively, with statistically significant difference (P<0.001). The diagnostic value of NLR in the EONS group (cutoff point, 1.24) showed 83.3% sensitivity and 93.3% specificity.
Conclusion
Eosinopenia has high specificity as a diagnostic marker for EONS and an increased NLR has high sensitivity and specificity as a diagnostic marker for EONS.
Keywords: Eosinophil count, Neutrophil to lymphocyte ratio, Early onset neonatal sepsis
Introduction
Neonatal sepsis is a clinical syndrome arising from the invasion of microorganisms into the bloodstream that arises in the first month of life [1]. Neonatal sepsis is still a major problem in neonatal care and still contributes significantly to disability and death. At least 1 million deaths per year occurring in the newborn period (0–28 days) are caused by infections, and 25% of infections cause newborn mortality, accounting for 10% of infant mortality worldwide [2,3]. Based on the time of occurrence, neonatal sepsis is divided into 2 types: early-onset neonatal sepsis (EONS), which occurs within the first 72 hours of life and late-onset neonatal sepsis (LONS), which occurs after 72 hours of life [1].
According to Indonesian Demographic Health Survey data in 2012, there were 32 deaths per 1000 live births [4]. In R.D. Kandou General Hospital Manado, during the period of January to December 2013, the incidence of infant mortality due to sepsis was as many as 127 cases (30.1%) out of a total of 421 babies [5].
The accurate and timely diagnosis of neonatal sepsis remains a challenging issue due to its nonspecific clinical presentation. The diagnosis criteria of EONS that we used in this study were based on at least 2 clinical criteria (hyper- or hypothermia, apnea or bradycardia spells, increased oxygen requirement, feeding intolerance, abdominal distension, lethargy and hypotonia, hypotension, or skin or subcutaneous lesions, such as petechial rash, abscesses, or sclerema) and 2 laboratory criteria (white blood cell [WBC] <5,000 cells/mm3, WBC >20,000 cells/mm3, immature-to-total neutrophil ratio [ITR] >0.2, platelet count <100,000 cells/mm3, CRP >10 mg/L) [6]. Evidence of infection from blood cultures itself often show insignificant results and takes too much time to obtain [7]. Efforts have been made to diagnose EONS using easy and cheap tools, such as eosinophil count and neutrophil-to-lymphocyte ratio (NLR) [8,9].
In previous studies, eosinopenia was shown to have good sensitivity and specificity in diagnosing both EONS and LONS, these studies used an adult sepsis cutoff point [8,9]. In this study, we try using a point of intersection for neonatal subjects. The diagnostic value of NLR for EONS alone in neonates has never been studied. The purpose of this study is to provide information bout the diagnostic value of eosinophil count and NLR for detecting EONS.
Materials and methods
A cross-sectional study was conducted in the Neonatology Ward of R.D. Kandou General Hospital Manado from July to October 2017. Study subjects were all neonates who met the inclusion criteria. We used a consecutive sampling method with a sample size of 120 neonates. Each study subject then had an anamnesis, a physical examination, and a laboratory examination (hematology profile, differential count, eosinophil count, and blood culture).
Included in the study were all neonates with possible EONS, born either vaginally or sectio caesaria and for whom parental consent for participation in this study had been obtained on a signed consent form. This study was conducted with the approval of the ethics committee of R.D. Kandou General Hospital Manado with the number 100/EC-KEPK/VII/2017. Postoperative neonates who had needed surgery due to congenital abnormalities were excluded from the study.
Neonates were suspected of having sepsis if they fulfilled 2 major criteria or 1 major criterion and 2 or more minor criteria. Septic risk factors included, as major risk factors, premature rupture of membranes >18 hours, intrapartum fever (>38°C), chorioamnionitis, greenish and foul smelling amniotic fluid, and fetal heart rate>160 beats/min. The minor risk factors consisted of premature rupture of membranes >12 hours, intrapartum fever (>37.5°C), low Apgar score (min 1 score <5 and min 5 score <7), very low birth weight (<1,500 g), vaginal discharge untreated, and suspected urinary tract infection in the mother[10-12].
Neonates were suspected of having EONS if there were 4 or more clinical symptoms and 2 or more hematologic profile abnormalities (with or without positive blood cultures). Clinical symptoms in neonates that indicated possible sepsis included lethargy, decrease in suction reflex, wheezing, irritability, seizures, bradycardia, apnea, tachypnea, oxygen saturation measured with pulse oxymeter <85%, paleness, decreased perfusion, hypothermia, hyperthermia, hypotonia, vomiting, diarrhea, ileus, bloating, feeding intolerance, elongated gastric emptying times, anemia, jaundice, petechiae, and purpura. Hematologic profile abnormalities included IT ratio ≥0.2, leukopenia (<5,000 cells/mm3 ), leukocytosis (>25,000 cells/mm3 ), or thrombocytopenia (<100,000 cells/mm3)[13].
Christensen et al. [14] in a retrospective study that was conducted in the United States from January 1, 2009, to May 31, 2009, with a sample size of 63,000 healthy neonates born at 22 to 42 weeks of gestation, obtained a normal eosinophil count range of 140–1,300 cells/mm3 with an average value of 550 cells/mm3.
The data were processed with IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Descriptive analyses were used to illustrate the characteristics of the data, receiver operating characteristic (ROC) analysis to assess the cutoff point of eosinophil count and neutrophil-to-lymphocyte ratio, and a chi-squared test to find the diagnostic value of eosinophil count and neutrophil-to-lymphocyte ratio by calculating the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results
There were 120 neonates suspected of having sepsis that fulfilled the inclusion criteria (Fig. 1). Table 1 shows the characteristics of our samples. Incidence of EONS was mostly found in males, 53 (59%), compared with females, 37 (41%). Blood cultures were made and resulted in 18 positive growth, consisting of Klebsiella pneumonia (9), Enterobacter aerogenes (3), Escherichia coli (1), Staphylococcus hominis (2), Staphylococcus saprophyticus (1), and Staphylococcus aureus (2).
Fig. 1.
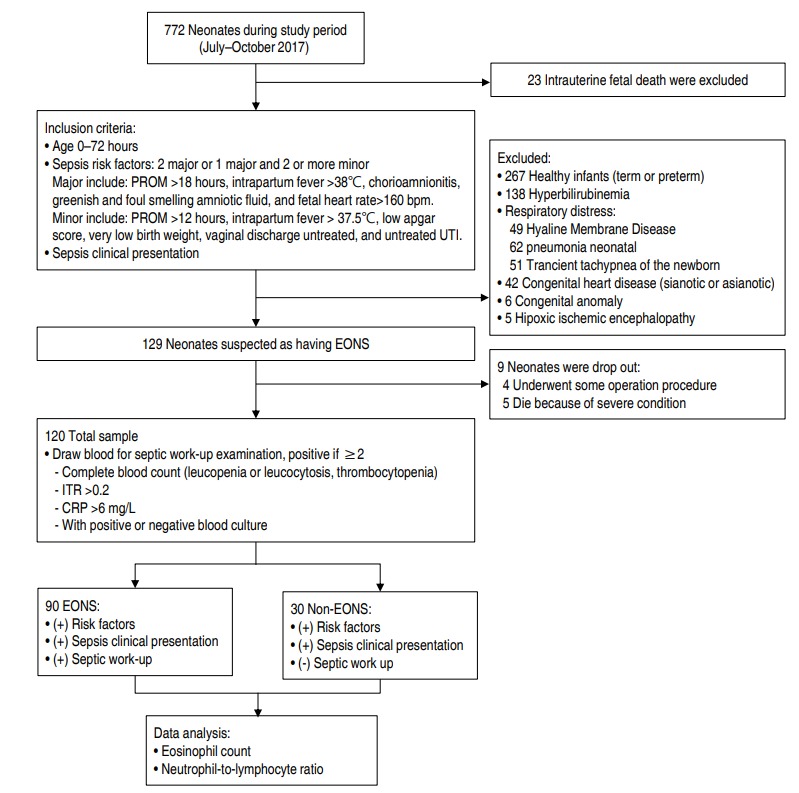
Study enrollment. Of 772 neonates admitted in the study period, 129 met early onset neonatal sepsis (EONS) inclusion criteria. Nine were dropouts, leaving a total of 120. After a septic work-up, 90 of 120 were placed in the EONS group. Analysis was performed to determine the relationship between EONS and the eosinophil count or neutrophil to lymphocyte ratio. PROM, premature rupture of membranes; UTI, urinary tract infection; ITR, immature-to-total neutrophil ratio; CRP, C-reactive protein.
Table 1.
Characteristics of study subjects (n=120)
| Characteristic | Value |
|---|---|
| Neonatal data | |
| Age (day) | |
| 0 | 54 (45.0) |
| 1 | 40 (33.3) |
| 2 | 26 (21.7) |
| Sex | |
| Male | 73 (60.8) |
| Female | 47 (39.2) |
| Birth weight (g) | 2,867±467 (1,450–4,100) |
| Maternal data | |
| Gestational age (wk) | |
| 28–33 weeks (preterm) | 9 (7.5) |
| 34–36 weeks (late preterm) | 15 (12.5) |
| 37–42 weeks (term) | 96 (80.0) |
| Sepsis risk factors | |
| Major | |
| Rupture of membrane>18 hours | 57 (47.5) |
| Maternal fever>38°C | 15 (12.5) |
| Chorioamnionitis | 8 (6.66) |
| Foul smelling liquor | 36 (30.0) |
| Sustained fetal heart rate>160 beats/min | 36 (30.0) |
| Minor | |
| Rupture of membrane>12 hours | 13 (10.8) |
| Maternal fever>37.5°C | 5 (4.2) |
| Low Apgar <5 at 1 min, <7 at 5 min | 17 (14.1) |
| Very low birth weight | 3 (2.5) |
| Prematurity | 18 (15.0) |
| Multiple gestation | 4 (3.3) |
| Untreated foul vaginal discharge | 52 (43.3) |
| Untreated urinary tract infection | 34 (28.3) |
Values are presented as number (%) or mean±standard deviation (range).
Mean eosinophil count from the EONS group was 169.8±197.1 cells/mm3, while that from the non-EONS group was 405.7±288.9 cells/mm3 with P<0.001 considered as significant. We also tallied their NLR and found that the mean NLR from the EONS and the non-EONS group was 2.82±2.29 and 0.82±0.32, respectively, also with P<0.001 considered as significant also. Fig. 2A shows the data distribution of eosinophil count in the EONS and the non-EONS group, while Fig. 2B shows the data distribution of NLR in both groups.
Fig. 2.
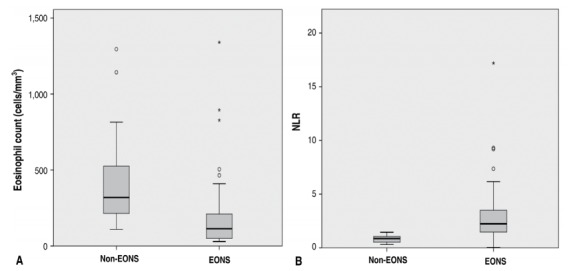
(A) Distribution of eosinophil count across EONS and non-EONS groups. Mean eosinophil count in the EONS group was 169.8±197.1 cells/mm3 and mean eosinophil count in the non-EONS group was 405.7±288.9 cells/mm3; P<0.0001. (B) Distribution of NLR across EONS and non-EONS group. Mean NLR in the EONS and non-EONS groups was 2.82±2.29 and 0.82±0.32, respectively; P<0.0001. EONS, early onset neonatal sepsis; NLR, neutrophil-tolymphocyte ratio.
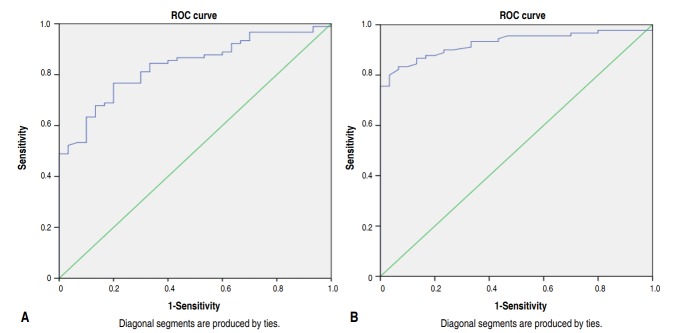
An ROC curve to analyze the ability of eosinophil count to diagnose EONS (Fig. 3A) demonstrates that with a cutoff of 140 cells/mm3, there is 60.0% sensitivity and 90.0% specificity. A similar analysis with NLR showed that the best specificity and sensitivity for EONS was at NLR 1.245 with sensitivity of 83.3% and specificity 93.3% (Fig. 3B).
Fig. 3.
(A) Receiver operating characteristic (ROC) curve predicting sensitivity and specificity of eosinophil count in detecting EONS. The ROC curve for eosinophil count demonstrates that with a cutoff of 140 cells/mm3, the sensitivity is about 60.0% and the specificity is 90.0%. (B) ROC curve predicting the sensitivity and specificity of NLR in detecting EONS. The ROC curve for NLR showed that the best specificity and sensitivity for EONS was at the cutoff of 1.245, with 83.3% sensitivity and 93.3% specificity.
Discussion
In our study, sepsis was found more in male than female neonates. In the EONS group, there were 90 neonates (75%), of which 53 (59%) were males. This result shows that male neonates are more susceptible to sepsis than female neonates. These findings are in accordance with a study in Denpasar which showed that neonatal sepsis incidence is higher in males (56.8%) than females (43.2%) [15]. Kardana also obtained neonatal sepsis incidence higher in males than in females [16].
In this study, in 90 neonates with EONS, there were only 18 positive bacterial growth found in blood cultures, 5 of which were bacteria: S. hominis (2), S. saprophyticus (1), and S. aureus (2). Thirteen were gram-negative bacteria, which are K. pneumoniae (9), E. coli (1), and E. aerogenes (3). The most common type of bacteria found was K. pneumoniae. Results obtained concerning the cause of EONS in previous research conducted by Bagus et al.17) at Dr. Soetomo Surabaya Hospital were: E. coli (19.1%), S. hominis (9.5%), K. pneumoniae (4.8%), E. aerogenes (4.8%), and S. saprophyticus (4.8%). The types of bacteria found were different from previous studies because there were different bacterial pattern variations present in the location of the study.
Traditionally, the definition of sepsis has included isolation of a pathogen from a normally sterile body fluid, such as blood or cerebrospinal fluid. The clinical features of sepsis can be induced by potent pro-inflammatory cytokines, and the term systemic inflammatory response syndrome has also been used when describing neonatal sepsis. For blood cultures, a minimum of 0.5–1 mL of blood should be obtained from 2 different venipunctures from 2 separate sites. Commonly used nonculture based diagnostic tests include total and differential WBC count, absolute and immature neutrophil counts, and the ratio of immature to total neutrophils. WBC count has limitations in terms of sensitivity. ITR>0.2 is suggestive of a bacterial infection and found to be predictive when used in combination with a complete blood cell obtained at more than 4 hours of age. The main benefit of WBC count is its NPV since normal serial values make it unlikely that a blood culture will be positive. WBC values are dynamic, so serial measurements over 24 hours might be more informative. CRP, procalcitonin, haptoglobin, fibrinogen, and inflammatory cytokines are also diagnostic tests that measure an inflammatory response [18].
Current evidence shows no one factor can be used to diagnose sepsis. However, promising results have been seen when 2 or more of these factors are combined. Due to the lack of consistent evidence in this area, no list of such factors has yet been developed. The best methods involved in the investigation of EONS is using the combination of maternal risk factors, clinical signs and symptoms, and various laboratory markers that are available. The gold standard for a definitive diagnosis is a blood culture. However, blood cultures can take as long as 48–72 hours, making them an unreliable tool in determining if treatment is needed in critical hours once the disease has begun. They also lack a high PPV, with less than 50% of the cases being positive. Blood cultures should taken before antibiotic therapy is initiated [19]. A negative blood culture does not exclude sepsis diagnosis as about 26% of all neonatal sepsis could be due to anaerobes. Furthermore, the etiological agent may not be isolated by media used in our study such as viral (e.g., rubella, cytomegalovirus), protozoal (e.g., Toxoplasma gondii), and treponemal (e.g., Treponema pallidum) pathogens. Earlier studies said that positive blood cultures only found 30%–40% of sepsis cases [20].
One of the important aspects of acute infection is the decline in eosinophil count that spreads through blood circulation quickly and persistently. Pathophysiology of eosinopenia during infection may be caused by the combination of the increased peripheral eosinophil sequestration due to localization of site infection, which can be caused by the increased drainage through lymphatic flow, a chemotaxis process in inflammation, an increase in peripheral eosinophil sequestration, pressure toward of process of eosinophil release from the bone marrow, and pressure toward eosinophil formation in bone marrow [21].
During bacterial sepsis, the bacteria’s endotoxin and lipopolysaccharide activate macrophage, neutrophil, and dendritic cells to release pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α, which will then activate the hypothalamus pituitary adrenal axis. The paraventricular nucleus in the anterior hypothalamus will release corticotropin-releasing hormone, which stimulates the anterior hypophysis to release adrenocorticotropin hormone (ACTH) into circulation. ACTH will stimulate the synthesis and release of glucocorticoid from the adrenal gland. Glucocorticoid will inhibit eosinophil release from the bone marrow, thus detaining eosinophil adhesion, migration, and chemotaxis through the inhibition of IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, chemokine and integrin, so that the amount of eosinophil will be reduced. This explains why the sepsis group has a lesser eosinophil count than the nonsepsis group [22,23].
Neutrophil and lymphocyte are important components of the immune system, which initially fights off infection [24]. During sepsis, a strong adhesion between neutrophil and endothelium was made, causing the failure of neutrophil to migrate to the infection site [25]. Moreover, bacterial products and cytokines released during sepsis also delay neutrophil apoptosis, which contributes to the degree of sepsis severity [26]. Neutrophil itself has a short lifespan, only about 24 hours. So, a sepsis patient who has trouble in the apoptosis process will have a prolonged neutrofil life in the blood. This is allegedly caused by nuclear factor-kappa B activation and the decrease of level 3 caspase [25,27]. Lymphocytopenia happens due to deployed lymphocyte into inflammation or infection site. In addition, lymphocyte apoptosis is significant in patients with sepsis. Lymphocytopenia as a sign of lymphocyte apoptosis is a part of the host’s normal immune response to stop and control an exaggerated immune response with the aim to stop further tissue damage. Within the first 24 hours of sepsis, lymphocytopenia happens because it was deployed from the blood circulation to the site of infection, which then leads to depletion of T cells CD4+ and CD8+ in the blood [28-30].
In this study, mean eosinofil count was 169.8±197.1 cells/mm3 from the group, and for the non-EONS group, it was 405.7± 288.9 cells/mm3. Based on the logistic regression analysis, a very significant correlation was found between eosinophil count and sepsis occurrence with P<0.001, where the lower the eosinophil count is, the higher the chance of sepsis. Furthermore, a very significant correlation was found between eosinophil count with EONS incidence in the point biserial result, with rpb=-0.419 and P<0.01. The ROC analysis showed AUC 83.5% with an eosinophil cutoff point of 140 cells/mm3 and sensitivity=60.0%, specificity=90.0%, PPV=94.7%, and NPV=42.9%. This cutoff point has an odds ratio (OR) of 13.5 (95% confidence interval [CI], 3.8–47.8).
This study also looked for NLR corellation with EONS, and we found that the mean NLR was 2.82±2.29 for the EONS group and 0.82±0.32 for the non-EONS group. Based on the logistic regression analysis, a very significant correlation was also found between NLR and sepsis incidence, where the higher the ratio, the higher the incidence of EONS, with a P-value <0.0001. Based on the ROC curve with a 1.245 cutoff point, the results show 83.3% sensitivity, 93.3% specificity, 94.7% PPV, and 65.2% NPV, with an OR=70.0 (95% CI, 15.0–325.8).
Our results are similar to those obtained by Bagus et al., [17] who conducted a study to compare the diagnostic values of immature granulocytes, eosinopenia, and IT ratios in detecting EONS in neonates (0–6 hours) with the risk of bacterial infection, where eosinopenia showed the highest specificity of detection for EONS. A study by Yefta et al. [31] also found that eosinophil percentage has a higher specificity than the sensitivity value in detecting neonatal sepsis.
Previous studies that have been done used a cutoff point from studies that were conducted on adults. A study with a cutoff point from neonatal samples has not yet been done. This study was performed with an eosinophil count cutoff point of 140 cells/mm3 for diagnosing EONS. NLR itself has never been performed to help diagnose EONS before. In this study, we used a cutoff point of 1.245.
This was the first study looking for any association between eosinophil count and NLR with incidence of EONS using cutoff points, sensitivity, and specificity from neonate subjects. One limitation of this study is that there was no follow-up examination in patients with higher eosinophil counts and low NLRs. To determine the risk of mortality in neonates with neonatal sepsis, serial examination of eosinophil and NLR is required.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Rusell AB, Isaacs D. Infection in the newborn. In: Rennie JM, Robertson NR, editors. Rennie & Roberton’s textbook of neonatology. 5th ed. London: Churchill Livingstone; 2012. pp. 1013–31. [Google Scholar]
- 2.Makkar M, Gupta C, Pathak R, Garg S, Mahajan NC. Performance evaluation of hematologic scoring system in early diagnosis of neonatal sepsis. J Clin Neonatol. 2013;2:25–9. doi: 10.4103/2249-4847.109243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 4.Jakarta (Indonesia): Health department of the Republic of Indonesia; 2013. Indonesia Demographic and Health Survey 2012 [Internet] [cited 2016 Mar 14]. Available from: https://www.dhsprogram.com/publications/publication-FR275-DHS-Final-Reports.cfm. [Google Scholar]
- 5.Manado (Indonesia): RSUP Dr. R. D. Kandou Manado; 2013. Prof Dr. R. D. Kandou [Internet] [cited 2017 Nov 4]. Available from: https://www.rsupkandou.com/profil. [Google Scholar]
- 6.Shrestha S, Dongol Singh S, Shrestha NC, Shrestha RP, Madhup SK. Comparision of clinical and laboratory parameters in culture proven and unproven early onset sepsis in NICU. Kathmandu Univ Med J (KUMJ) 2013;11:310–4. doi: 10.3126/kumj.v11i4.12528. [DOI] [PubMed] [Google Scholar]
- 7.Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010;10:39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12:R59. doi: 10.1186/cc6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaaban H, Daniel S, Sison R, Slim J, Perez G. Eosinopenia: is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J Crit Care. 2010;25:570–5. doi: 10.1016/j.jcrc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Aminullah A. Sepsis in newborn. In: Kosim MS, Yunanto A, Dewi R, Sarosa GI, Usman A, editors. Textbook of neonatology. Jakarta (Indonesia): Indonesian of Pediatric Society; 2012. pp. 170–87. [Google Scholar]
- 11.Rohsiswatmo R, Dewanto NE, Rizalya D. Sepsis in neonates. Jakarta (Indonesia): Indonesian of Pediatric Society; 2010. pp. 107–87. [Google Scholar]
- 12.Gomella TL, Cunningham MD, Eyal FG, Zenk KE. Sepsis. In: Gomella TL, Cunningham MD, Eyal FG, editors. Neonatology: management, procedures, on-call problems, diseases, and drugs. 7th ed. New York: McGraw Hill; 2013. pp. 865–73. [Google Scholar]
- 13.Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med. 2005;6(3 Suppl):S45–9. doi: 10.1097/01.PCC.0000161946.73305.0A. [DOI] [PubMed] [Google Scholar]
- 14.Christensen RD, Jensen J, Maheshwari A, Henry E. Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63 000 records in a multihospital health-care system. J Perinatol. 2010;30:540–5. doi: 10.1038/jp.2009.196. [DOI] [PubMed] [Google Scholar]
- 15.Putra PJ. Incidence and factors associated with neonatal sepsis at Sanglah General Hospital in Denpasar. Sari Pediatr. 2012;14:205–10. [Google Scholar]
- 16.Kardana IM. Incidence and factors associated with mortality of neonatal sepsis. Paediatr Indones. 2011;51:144–8. [Google Scholar]
- 17.Bagus E, Kahar H, Wardhani P. Diagnostic values of immature granulocytes, eosinopenia and I/T ratio in detection of early onset neonatal sepsis in neonates with bacterial infection risk. Folia Medika Indonesina. 2014;50:43–7. [Google Scholar]
- 18.Klobucar B. Diagnostic criteria for early onset neonatal sepsis (unpublished graduate thesis) Zagreb (Croatia): University of Zagreb School of Medicine; 2017. [Google Scholar]
- 19.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–80. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 20.Ferrieri P, Wallen LD. Neonatal bacterial sepsis. In: Gleason CA, Devaskar SU, editors. Avery’s disease of the newborn. 9th ed. Philadelphia (PA): Saunders Elsevier; 2012. pp. 538–50. [Google Scholar]
- 21.El-Din EM, El-Sokkary MM, Bassiouny MR, Hassan R. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed Res Int. 2015 doi: 10.1155/2015/509484. Article ID 509484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980;65:1265–71. doi: 10.1172/JCI109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guntur AH. SIRS and sepsis: Immunology, diagnosis and treatment. Surakarta: Sebelas Maret University Press; 2006. pp. 1–16. [Google Scholar]
- 24.Luhulima D, Hidayati W, Sri Rejeki IG, Permatasari R. Eosinopenia and procalsitonin in sepsis. Indones J Clin Pathol Med Lab. 2013;19:1–11. [Google Scholar]
- 25.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Monneret G, Payen D. Sepsis induced immunosuppression: from cellular dysfunction to immunotherapy. Nat Rev Immunol. 2013;13:862–72. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–8. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 30.Shubin NJ, Monaghan SF, Heffernan DS, Chung CS, Ayala A. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 2013;17:R276. doi: 10.1186/cc13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yefta EK, Yuniati T, Rahayuningsih SE. The validity of eosinopenia as a marker of diagnosis in bacterial neonatal sepsis. Med Indones. 2009;59:601–6. [Google Scholar]