Fig. 1. Sxph structure.
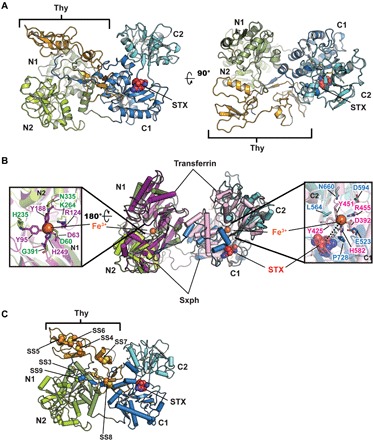
(A) R. catesbeiana Sxph:STX: complex ribbon diagram. Domains are indicated and are colored as follows: N1 (smudge), N2 (limon), thyroglobulin (Thy; bright orange), C1 (marine), and C2 (cyan). STX (red) is shown as space filling. (B) Superposition of Sxph and rabbit transferrin [Protein Data Bank (PDB): 1JNF] (32). Transferrin N-lobe and C-lobe are colored purple and pink, respectively. Sxph Thy1 repeats are not shown. Insets show transferrin Fe3+ ligands and Sxph equivalents as sticks. N domain: transferrin (purple) and Sxph (green); C domain: transferrin (pink) and Sxph (blue). STX (red) is shown as space filling. Right hand inset shows distance between the STX center and transferrin Fe3+. (C) Cartoon diagram showing unique Sxph disulfide bonds in space filling representation: SS3 (Cys27 to Cys417) SS4 (Cys91 to Cys111), SS5 (Cys122 to Cys129), SS6 (Cys131 to Cys153), SS7 (Cys161 to Cys183), SS8 (Cys203 to Cys225), and SS9 (Cys234 to Cys825). Colors and labels are the same as in (A).
