Abstract
Background:
Patient-reported outcome measures (PROMs) have become widely adopted in the care of patients with prostate cancer, but there is no validated crosswalk between two commonly used instruments, the Expanded Prostate Cancer Index Composite Short Form (EPIC-26) and the Memorial Sloan Kettering (MSK) instrument, which consists of the International Index of Erectile Function-6 (IIEF-6) questionnaire and the MSK radical prostatectomy urinary outcome scale.
Objective:
To develop and validate bidirectional crosswalks between the sexual and urinary domains (single domain in MSK, separate incontinence and irritative/obstructive domains in EPIC-26) of the MSK and EPIC-26 instruments.
Design, setting, and participants:
Radical prostatectomy (RP) patients completing instruments at MSK and Michigan Urological Surgery Improvement Collaborative (MUSIC) between January and May of 2017 were invited to enroll. Stratified random sampling (by institution, MSK urinary function score, and MSK erectile function score) was used to divide patient data into training and test sets. Models were developed to predict the domain score for each instrument using the other’s item responses and domain scores. Performance was evaluated using capped root-mean-squared error and accuracy at established thresholds.
Results and limitations:
We received 517 instruments at MSK and 1033 within MUSIC, which were assigned to training (825), post-RP test (412), and pre-RP test (313) sets. We found the crosswalks to have low error and high accuracy. Although the crosswalks are more accurate if responses to each item are known, it is possible to convert between instruments on the basis of a total domain score.
Conclusions:
The crosswalks are a valid way to convert between sexual and urinary domains of the MSK and EPIC-26 instruments.
Keywords: Patient-reported outcomes, Sexual dysfunction, Urinary incontinence
Patient summary:
We developed and validated a set of formulas that allow conversion of sexual and urinary function scores between the Memorial Sloan Kettering and Expanded Prostate Cancer Index Composite Short Form patient-reported outcome questionnaires. These crosswalks allow seamless transition between the two questionnaires.
1. Introduction
Patient-reported outcome measures (PROMs) have become widely adopted in the care of patients with localized prostate cancer [1,2]. This trend has been driven by increased recognition of the importance of patient preferences in the shared decision-making process, evidence demonstrating that use of PROMs improves outcomes for patients with cancer, and technological advances that allow delivery of the tools through a web-based platform [3–5]. One of the first validated PROMs for localized prostate cancer delivered through a web-based platform was developed at the Memorial Sloan Kettering Cancer Center (MSK) [3]. It was first administered via the Symptom Tracking and Reporting (STAR) system at MSK in 2009, and the Michigan Urological Surgery Improvement Collaborative (MUSIC) adopted it in 2014 to assess health-related quality of life in patients following radical prostatectomy (RP) [3,4]. Both groups have used the MSK instrument to study contemporary long-term outcomes in patients who have undergone RP for localized prostate cancer [6,7].
Several other instruments have also been developed to assess PROMs in prostate cancer patients, and in 2015 a working group convened by the International Consortium for Health Outcomes Measurement (ICHOM) recommended use of the Expanded Prostate Cancer Index Composite Short Form (EPIC-26) over other instruments [8]. The ICHOM noted in its report that the choice to select a single instrument was “a contentious decision because centers of excellence have established prospective registries using various measures in [prostate cancer], and there is no compelling evidence for the advantage of one particular PROM over another.” Because PROMs are often used to study long-term outcomes, health systems may consider the loss of information on switching PROM instruments an insurmountable barrier. Research studies or quality assurance initiatives may include PROMs measured using different instruments, making it difficult for investigators to compare results. It is not clear, for instance, whether a score of 80 out of 100 on the EPIC sexual function scale is better or worse than a score of 24 out of 30 in the MSK erectile function domain. Converting from one PROM scale to another is known as a “crosswalk”. A previous study reported different crosswalks for converting between EPIC-26, the UCLA Prostate Cancer Index (PCI), and the Sexual Health Inventory for Men (SHIM), but did not evaluate conversions between the MSK and EPIC-26 instruments [9]. In this study, we created bidirectional crosswalks for converting urinary and sexual domain scores between the MSK and EPIC-26 instruments in post-RP patients. We validated the crosswalks in sets of both pre-RP and post-RP patients.
2. Patients and methods
2.1. Data source
The data for this study were prospectively collected from MSK and MUSIC. MUSIC is a quality improvement collaborative that was established in 2011 in partnership with Blue Cross Blue Shield of Michigan and currently comprises 44 diverse community and academic urology practices representing approximately 90% of the urologists in the state of Michigan. The MUSIC data collection and validation practices are described in the Supplementary material. The MSK institutional review board (IRB) approved the inclusion of MSK patients in this research study. Each MUSIC practice obtained an exemption or approval for collaborative participation from a local IRB.
2.2. Study cohort
All patients scheduled for RP who completed quality-of-life questionnaires at MSK between March and June of 2017 and within MUSIC between January and May of 2017 as part of routine care were invited to join the study. Additional recruitment details are provided in the Supplementary material. To create training and validation data sets, we used a 2:1 random sampling for post-RP questionnaires after stratifying by institution, MSK urinary function score, and MSK erectile function score. Stratification was used to ensure a similar distribution of patients in both data sets. As a sensitivity analysis, we evaluated the performance of the crosswalks separately using a cohort of pre-RP patients. These were not included in either the training or test sets because of concerns that inclusion would lead to a ceiling effect in model fitting and artificially improve the model performance.
2.3. Description of the instruments
The MSK instrument includes assessments of sexual (using the International Index of Erectile Function-6 [IEEF-6] instrument) and urinary domains, questions about the use of oral and injectable erectile medication, a question on hernias, and a question on general quality of life. The EPIC-26 instrument includes five domains: sexual, urinary incontinence (UIN), urinary irritative/obstructive (UIR), bowel, and hormonal domains. For this study, we focused on the sexual and urinary domains of the MSK instrument and the sexual, urinary incontinence, and urinary irritative/obstructive domains of the EPIC-26 instrument. A complete list of questions for the relevant domains of both instruments is presented in Supplementary Table 1.
2.4. Development of the crosswalk models
The aim of this study was to create bidirectional crosswalks for each of the domains, allowing for EPIC-26 scores to be converted into their MSK equivalents and vice versa. One complicating factor is that the MSK instrument has a single urinary domain, whereas EPIC-26 has a separate domain scores for UIN and UIR symptoms. We considered three scenarios when developing the crosswalk models. First, we considered a scenario in which there is a need to convert a domain score from one instrument to the other instrument, but only total scores are available and no data for individual questions. This might be the case, for instance, when comparing two results reported in the literature. Second, we considered a scenario in which an individual’s responses for every item are available, so that all items in a domain (or a subset of items) can be used to predict the domain score for the other instrument. This would be the case, for example, in a collaborative research or quality assurance initiative. Third, we considered a scenario in which domain scores have been converted into binary endpoints (eg, erectile function vs dysfunction), which is often important in quality assurance programs. MSK already has established binary definitions of potency and urinary continence, so we evaluated how these could be best translated to EPIC-26 thresholds. The MSK IIEF-6 potency threshold of 24 was established on the basis of a multistep validation process, and the MSK urinary continence threshold of 17 was established according to clinical judgment and has been in use at MSK since 2009 and at MUSIC since 2014 [4,10].
Models were developed for each of the crosswalks independently by investigators at MSK and MUSIC using the training set. Multiple types of models were considered, including linear models, decision trees, gradient-boosted machines, and random forests.
2.5. Validation of the crosswalk models
The performance of each of the crosswalk models was measured in the post-RP and pre-RP patient cohorts. The prediction error was measured as the capped root-mean-squared error (RMSE), whereby predicted values that were outside the valid range of the outcome variable were first “capped” to the closest valid value (eg, a predicted value of 105 for EPIC domains with a valid range of 0–100 was converted to 100), and then the RMSE was calculated in typical fashion for unchanged and capped values.
Accuracy was calculated by converting the predicted score into a binary endpoint according to a selected threshold and then dividing the sum of true positives and true negatives by the total number of patients. For MSK, we calculated accuracy for the sexual and urinary function domains using established thresholds for potency (≥24 out of 30) and continence (≥17 out of 21), respectively. For EPIC, we used the model with the lowest RMSE to translate the established MSK thresholds into EPIC thresholds. These thresholds were then used to calculate the accuracy of the models predicting sexual function and UIN. As there is no accepted threshold for UIR symptoms, no accuracy was computed for this domain. Selection of the final models is described in the Supplementary material.
2.6. Model calibration
We assessed calibration of the final crosswalk models by plotting predicted versus actual domain scores and then qualitatively evaluating whether the mean predicted and actual values were similar across all valid values for each domain [11].
2.7. Missing data
For each direction for each crosswalk, we only used instruments with complete data available for the variable included.
2.8. Statistical analysis
Statistical analysis was performed independently at MSK and MUSIC using Stata v.15.0 (StataCorp, College Station, TX, USA) and R v.3.4.3 (R Foundation for Statistical Computing, Vienna, Austria), respectively.
3. Results
We received 517 concurrently completed instruments at MSK and 1033 such instruments in MUSIC. Of these, 1237 instruments were completed post-RP and were assigned to a training set (825) and a post-RP test set (412) using 2:1 stratified random sampling. The remaining 313 instruments, all from MUSIC, were completed pre-RP and were evaluated separately. The baseline characteristics of the training and test sets are presented in Table 1 and the distribution of instrument responses in the training and post-RP test sets is presented in Table 2. Within the test set, the distribution of instrument items stratified by pre-RP and post-RP is presented in Supplementary Table 2. As expected, the distribution of items before and after surgery is quite different, with patients generally experiencing higher function before RP. For models predicting MSK domain scores, we evaluated the capped RMSE and accuracy using the established thresholds for sexual domain scores and urinary function. After considering all three scenarios (domain-to-domain, items-to-domain, and accuracy), we achieved consensus on choice of the final models. Although we considered multiple model types, we selected linear models for the crosswalk because their performance was similar to that of nonlinear models (Supplementary Table 3) and we had concerns about the ease of dissemination and adoption of nonlinear models. We used the final models to calculate the EPIC-26 thresholds for potency (≥73 out of 100 on the EPIC-26 sexual domain score) and urinary continence (≥74 out of 100 on the EPIC UIN domain score).
Table 1.
Baseline characteristics in the training and test sets
| Characteristic | Training set (N = 825; 53%) |
Post-RP test set (N = 412; 27%) |
Pre-RP test set (N = 313; 20%) |
|---|---|---|---|
| Mean age, yr (standard deviation) | 64 (7.0) | 64 (7.0) | 63 (7.0) |
| Race, n (%) | |||
| Caucasian | 702 (85) | 351 (85) | 231 (74) |
| African American | 56 (6.8) | 33 (8.0) | 23 (7.3) |
| Asian | 15 (1.8) | 3 (0.7) | 2 (0.6) |
| Other | 5 (0.6) | 3 (0.7) | 3 (1.0) |
| Unknown | 47 (5.7) | 22 (5.3) | 54 (17) |
| Organization, n (%) | |||
| Memorial Sloan Kettering | 345 (42) | 172 (24) | 0 (0) |
| MUSIC | 480 (58) | 240 (58) | 313 (100) |
| Time since RP, n (%) | |||
| 0 mo | 0 (0) | 0 (0) | 313 (100) |
| 3 mo | 205 (25) | 114 (28) | 0 (0) |
| 6 mo | 175 (21) | 76 (18) | 0 (0) |
| 12 mo | 266 (32) | 100 (24) | 0 (0) |
| 24 mo | 114 (14) | 81 (20) | 0 (0) |
| ≥36 mo | 65 (7.9) | 41 (10) | 0 (0) |
| Gleason grade group, n (%) | |||
| 1 (GS ≤6) | 75 (9.1) | 35 (8.5) | 19 (6.1) |
| 2 (GS 3 + 4) | 444 (54) | 198 (48) | 177 (57) |
| 3 (GS 4 + 3) | 171 (21) | 107 (26) | 62 (20) |
| 4 (GS 8) | 56 (6.8) | 22 (5.3) | 12 (3.8) |
| 5 (GS 9–10) | 69 (8.4) | 41 (10) | 23 (7.3) |
| Unknown | 10 (1.2) | 9 (2.2) | 20 (6.4) |
RP = radical prostatectomy; MUSIC = Michigan Urological Surgery Improvement Collaborative; GS = Gleason score.
Table 2.
Distribution of instrument items in the training and post-RP test sets
| Instrument item | Training set |
Post-RP test set |
||
|---|---|---|---|---|
| N = 825 | Mean (SD) | N = 412 | Mean (SD) | |
| MSK sexual domain (IIEF-6) | 689 | 11 (10) | 346 | 11 (9.0) |
| MSK urinary domain | 807 | 16 (5.0) | 403 | 16 (5.0) |
| EPIC sexual domain | 782 | 38 (28) | 391 | 37 (29) |
| EPIC UIN domain | 809 | 69 (28) | 400 | 66 (27) |
| EPIC UIR domain | 797 | 89 (14) | 395 | 90 (12) |
| MSK sexual domain (IIEF-6) | ||||
| MSKEF2 | 688 | 2.3 (1.3) | 346 | 2.2 (1.3) |
| MSKEF3 | 688 | 3.2 (1.7) | 348 | 3.1 (1.7) |
| MSKEF4 | 675 | 2.9 (1.7) | 341 | 2.8 (1.7) |
| MSKEF5 | 802 | 2.3 (1.8) | 398 | 2.3 (1.8) |
| MSKEF6 | 788 | 2.3 (1.8) | 392 | 2.2 (1.8) |
| MSKEF7 | 771 | 2.4 (1.9) | 378 | 2.3 (1.8) |
| MSK urinary domain | ||||
| MSKUF1 | 806 | 2.2 (1.4) | 403 | 2.2 (1.4) |
| MSKUF2 | 806 | 1.9 (1.3) | 401 | 1.9 (1.4) |
| MSKUF3 | 805 | 1.7 (1.1) | 401 | 1.7 (1.2) |
| MSKUF4 | 805 | 2.2 (1.2) | 402 | 2.1 (1.2) |
| MSKUF5 | 804 | 2.1 (1.2) | 401 | 2.2 (1.2) |
| EPIC sexual domain | ||||
| EPIC57 | 765 | 2.1 (1.3) | 380 | 2.1 (1.3) |
| EPIC58 | 774 | 2.8 (1.4) | 389 | 2.7 (1.4) |
| EPIC59 | 800 | 2.2 (1.2) | 399 | 2.2 (1.2) |
| EPIC60 | 797 | 2.6 (1.3) | 400 | 2.5 (1.3) |
| EPIC64 | 798 | 2.2 (1.3) | 400 | 2.1 (1.3) |
| EPIC68 | 799 | 3.2 (1.4) | 401 | 3.2 (1.4) |
| EPIC UIN | ||||
| EPIC23 | 810 | 3.2 (1.7) | 402 | 3.1 (1.6) |
| EPIC26 | 810 | 3.2 (0.7) | 402 | 3.1 (0.7) |
| EPIC27 | 810 | 0.72 (0.96) | 402 | 0.72 (0.97) |
| EPIC28 | 809 | 1.2 (1.1) | 400 | 1.3 (1.2) |
| EPIC UIR | ||||
| EPIC29 | 807 | 0.16 (0.56) | 397 | 0.15 (0.51) |
| EPIC30 | 806 | 0.05 (0.36) | 398 | 0.02 (0.12) |
| EPIC31 | 802 | 0.48 (0.92) | 399 | 0.40 (0.80) |
| EPIC33 | 808 | 1.1 (1.2) | 398 | 1.1 (1.2) |
| EPIC urinary overall (EPIC34) | 809 | 2.2 (1.2) | 400 | 2.2 (1.2) |
RP = radical prostatectomy; SD = standard deviation; MSK = Memorial Sloan Kettering; EPIC = Expanded Prostate Cancer Index Composite; IIEF = International Index of Erectile Function; UIN = urinary incontinence; UIR urinary irritative/obstructive.
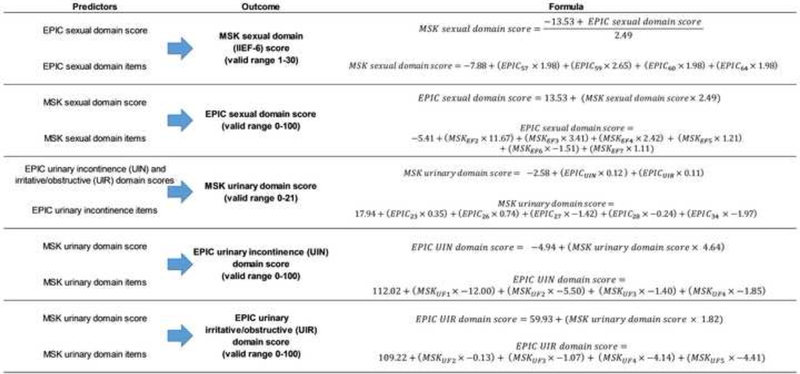
We found that bidirectional crosswalks between the MSK and EPIC thresholds were at least 80% accurate across all models at a clinically relevant threshold, with low error in the post-RP test set (Table 3). The crosswalks performed similarly to slightly better in the pre-RP compared to the post-RP test set for prediction of the MSK urinary and EPIC-26 UIN domain scores (Supplementary Table 4). On the contrary, the crosswalks predicting sexual domain and UIR domain scores exhibited better performance in the post-RP than in the pre-RP test set. The final model formulas are presented in Figure 1. For the sexual function domain, the crosswalk formulas in the two directions are simple mathematical transformations of one another. For all other crosswalks, separate models exist for each direction.
Table 3.
Performance of the final models on the post-radical prostatectomy test set
| Predictors | Outcome | Capped RMSE | Accuracya |
|---|---|---|---|
| EPIC-26 sexual domain score | MSK sexual domain (IIEF-6) | 6.1 | 0.90 |
| EPIC-26 sexual domain items | score (valid range 1–30) | 6.0 | 0.93 |
| MSK sexual domain (IIEF-6) score | EPIC-26 sexual domain score | 17 | 0.90 |
| MSK sexual domain (IIEF-6) items | (valid range 0–100) | 14 | 0.91 |
| EPIC-26 UIN and UIR domain scores | MSK urinary domain score (valid range 0–21) | 2.3 | 0.80 |
| EPIC-26 UIN items + overall item | 2.3 | 0.83 | |
| MSK urinary domain score | EPIC-26 UIN domain score | 14 | 0.82 |
| MSK urinary domain items | (valid range 0–100) | 12 | 0.82 |
| MSK urinary domain score | EPIC-26 UIR domain score | 9 | NA |
| MSK urinary domain items | (valid range 0–100) | 9 | NA |
RMSE = root mean square error; EPIC = Expanded Prostate Cancer Index Composite; MSK = Memorial Sloan Kettering; IIEF = International Index of Erectile Function; UIN = urinary incontinence; UIR urinary irritative/obstructive; NA = not applicable.
Accuracy is assessed after conversion of outcome variables into binary endpoints. In MSK, potency is defined as ≥24 (out of 30) and urinary continence is defined as ≥17 (out of 21). These values have been used extensively in the scientific literature. EPIC-26 cutpoints are calculated using the crosswalk formulas from the accepted MSK values. In EPIC, potency is defined as ≥73 (out of 100) and urinary continence is defined as ≥74 (out of 100). No cutoffs are proposed for the EPIC-26 urinary irritative/obstructive domain.
Fig. 1.
Formulas for the final crosswalk models.
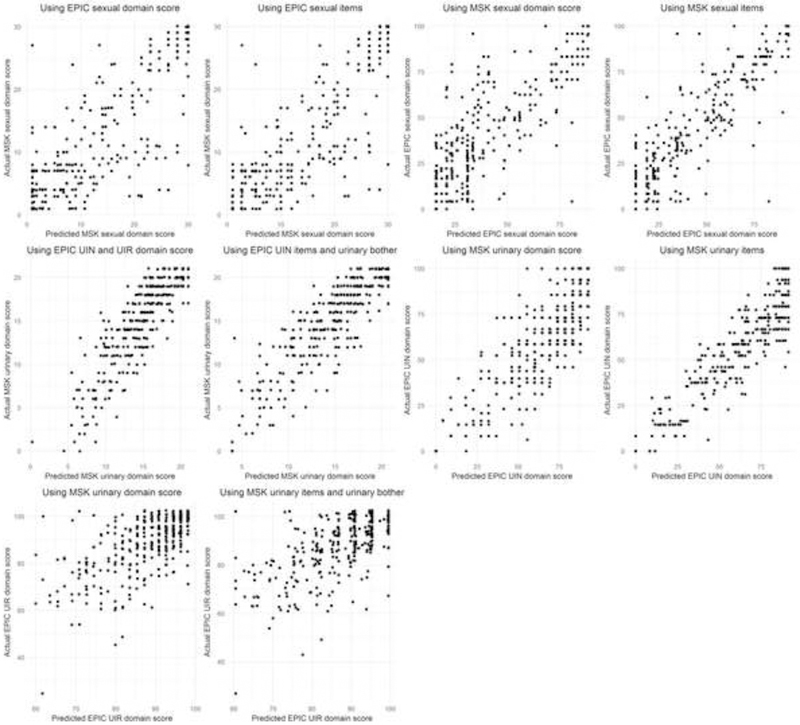
The final models appear to be generally well calibrated in the post-RP test set (Fig. 2), but crosswalks for the sexual domains exhibit high variance in the pre-RP test set (Supplementary Fig. 1).
Fig. 2.
Scatter plots comparing predicted versus actual domain scores to assess model calibration in the post–radical prostatectomy test set. It should be noted that the points have been slightly shifted randomly to minimize overlap.
MSK = Memorial Sloan Kettering; EPIC = Expanded Prostate Cancer Index Composite; UIN = urinary incontinence; UIR = urinary irritative/obstructive.
4. Discussion
We developed crosswalks for the sexual and urinary domains of the MSK and EPIC-26 instruments. We validated the crosswalks in a random sample of patients with localized prostate cancer at MSK and in MUSIC and found that they had low errors and a high level of accuracy for post-RP patients—above 80% at clinically relevant thresholds—and minimal bias. We established new thresholds for potency and continence in EPIC-26 and found that these generally concurred with established thresholds for the MSK instrument. On the basis of our results, we formulated a set of recommendations for use of the crosswalks (Table 4). We expected to find that the crosswalks would perform similarly or better among pre-RP patients as compared to post-RP patients because pre-RP patients generally have higher domain scores in a narrower range, but we found that this was only true for predicting the MSK urinary and EPIC-26 UIN domains. The remaining crosswalks had lower error and higher accuracy among post-RP patients. This difference was particularly pronounced in predicting the EPIC-26 UIR domain score.
Table 4.
Recommendations for use of the crosswalks
| Recommendation |
|---|
|
MSK = Memorial Sloan Kettering; EPIC = Expanded Prostate Cancer Index Composite; IIEF = International Index of Erectile Function.
Adoption of a single method for PROM ascertainment is well intentioned, but can cause a number of practical problems, including backwards compatibility with data collected via other instruments. Crosswalks are a solution to these problems. Previous crosswalks have addressed conversions between EPIC-26, the UCLA-PCI, and the SHIM instruments, although none of these crosswalks have addressed UIR symptoms, which are of particular relevance in patients treated with localized radiation therapy [9]. Because EPIC-26 was developed to expand the scope of the UCLA-PCI, the two instruments have several questions in common, although the UCLA-PCI instrument lacks a UIR domain. Although the MSK instrument does not address UIR symptoms, as it was developed for use in radical prostatectomy patients, the crosswalk from MSK urinary items to EPIC-26 UIR domain scores performs well. Somewhat surprisingly, the MSK urinary domain score predicts the EPIC-26 UIR domain with lower error than the UIN domain, but this may be because of the lower overall variance for UIR domain scores. Conversely, the lowest error in predicting the MSK urinary domain arises from using both EPIC UIN and UIR as predictors rather than either one alone. This suggests that the MSK urinary domain can be predicted by both UIN and UIR symptoms.
Our study should be considered in the context of several limitations. First, the instruments were administered during a relatively short time period. While certain patients did complete the instruments at multiple time points following RP, we did not have an adequate sample size to evaluate the fidelity of the crosswalk longitudinally in individual patients. Second, our data are drawn only from patients undergoing RP, so we cannot necessarily generalize use of the crosswalks to patients undergoing other treatments. Lastly, our data are drawn from a primarily Caucasian population, which could limit its generalizability to racial minorities. Prior literature demonstrating the validity of EPIC-26 in racially diverse cohorts does not suggest that the crosswalk would lose validity in such populations [12–15].
Despite these limitations, our study has important implications. The crosswalk between the MSK and EPIC-26 instruments is the first to address sexual function, UIN, and UIR symptoms in patients with prostate cancer. We have established cutoffs for potency and UIN in EPIC-26 that are concordant with thresholds established at MSK that have been widely adopted, which may be useful to surgeons and researchers interested in tracking PROMs longitudinally at the level of a surgeon or practice. Combining PROMs from multiple institutions that use different instruments could facilitate large-scale comparative effectiveness research, although gains in sample size may be partly offset by the introduction of measurement error.
In the future, we plan to integrate data from the MSK and EPIC-26 instruments to better understand the effects of ongoing interventions focused on improving PROMs.
5. Conclusions
We developed and validated bidirectional crosswalks to allow conversion between sexual and urinary domain items and scores from the MSK and EPIC-26 instruments. These crosswalks provide practices that use the IIEF-6 and MSK urinary instruments with a feasible way to transition to the EPIC-26 instrument in accordance with ICHOM recommendations and vice versa. They also allow prostate cancer researchers across institutions to aggregate and learn from PROMs data in ways not previously possible.
Supplementary Material
Acknowledgments:
The corresponding author would like to thank the Movember Foundation for support of this project and the support staff from Memorial Sloan Kettering and the Michigan Urological Surgery Improvement Collaborative.
Funding/Support and role of the sponsor: This study was supported in part by an NIH/NCI Cancer Center Support Grant to MSKCC under award number P30 CA00874 and grant P50-CA92629 SPORE from the National Cancer Institute. The sponsors played no direct role in the study.
Financial disclosures: Karandeep Singh certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Karandeep Singh received grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (grant 5K12DK111011) and salary support from Blue Cross Blue Shield of Michigan. Andrew J. Vickers received a grant from the Movember Foundation to develop this crosswalk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We developed and validated a set of formulas that allow conversion of sexual and urinary function scores between the Memorial Sloan Kettering and Expanded Prostate Cancer Index Composite Short Form (EPIC-26) patient-reported outcome questionnaires. These crosswalks allow seamless transition between the two questionnaires.
References
- 1.Atherton PJ, Sloan JA. Rising importance of patient-reported outcomes. Lancet Oncol 2006;7:883–4. 10.1016/S1470-2045(06)70914-7 [DOI] [PubMed] [Google Scholar]
- 2.Protopapa E, van der Meulen J, Moore CM, Smith SC. Patient-reported outcome (PRO) questionnaires for men who have radical surgery for prostate cancer: a conceptual review of existing instruments. BJU Int 2017;120:468–81. 10.1111/bju.13896 [DOI] [PubMed] [Google Scholar]
- 3.Vickers AJ, Savage CJ, Shouery M, Eastham JA, Scardino PT, Basch EM. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes 2010;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas SM, Kim TK, Ghani KR, et al. Establishment of a web-based system for collection of patient-reported outcomes after radical prostatectomy in a statewide quality improvement collaborative. Urology 2017;107:96–102. 10.1016/j.urology.2017.04.058 [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–8. 10.1001/jama.2017.7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JK, Assel M, Thong AE, et al. Unexpected long-term improvements in urinary and erectile function in a large cohort of men with self-reported outcomes following radical prostatectomy. Eur Urol 2015;68:899–905. 10.1016/j.eururo.2015.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon M, Dalela D, Jamil M, et al. Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the Retzius sparing and standard approaches. J Urol 2018;199:1210–7. 10.1016/j.juro.2017.11.115 [DOI] [PubMed] [Google Scholar]
- 8.Morgans AK, van Bommel AC, Stowell C, et al. Development of a standardized set of patient-centered outcomes for advanced prostate cancer: an international effort for a unified approach. Eur Urol 2015;68:891–8. 10.1016/j.eururo.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 9.Vertosick EA, Vickers AJ, Cowan JE, Broering JM, Carroll PR, Cooperberg MR. Interpreting patient reported urinary and sexual function outcomes across multiple validated instruments. J Urol 2017;198:671–7. 10.1016/j.juro.2017.03.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terrier JE, Mulhall JP, Nelson CJ. Exploring the optimal erectile function domain score cutoff that defines sexual satisfaction after radical prostatectomy. J Sex Med 2017;14:804–9. 10.1016/j.jsxm.2017.04.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology 2010;21:128–38. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrer M, Garin O, Pera J, et al. [Evaluation of the quality of life of patients with localized prostate cancer: validation of the Spanish version of the EPIC]. Med Clin 2009;132:128–35. 10.1016/j.medcli.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Vigneault É, Savard J, Savard M-H, et al. Validation of the French-Canadian version of the Expanded Prostate Cancer Index Composite (EPIC) in a French-Canadian population. Can Urol Assoc J 2017;11:404–10. 10.5489/cuaj.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umbehr MH, Bachmann LM, Poyet C, et al. The German version of the Expanded Prostate Cancer Index Composite (EPIC): translation, validation and minimal important difference estimation. Health Qual Life Outcomes 2018;16:36 10.1186/s12955-018-0859-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam WWT, Tse MA, Ng CNL, Chung EKM, Fielding R. Psychometric assessment of the Chinese version of the abbreviated Expanded Prostate Cancer Index Composite (EPIC-26) and the clinical practice version (EPIC-CP) in Chinese men with prostate cancer. J Pain Symptom Manage 2017;53:1085–90. 10.1016/j.jpainsymman.2017.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.