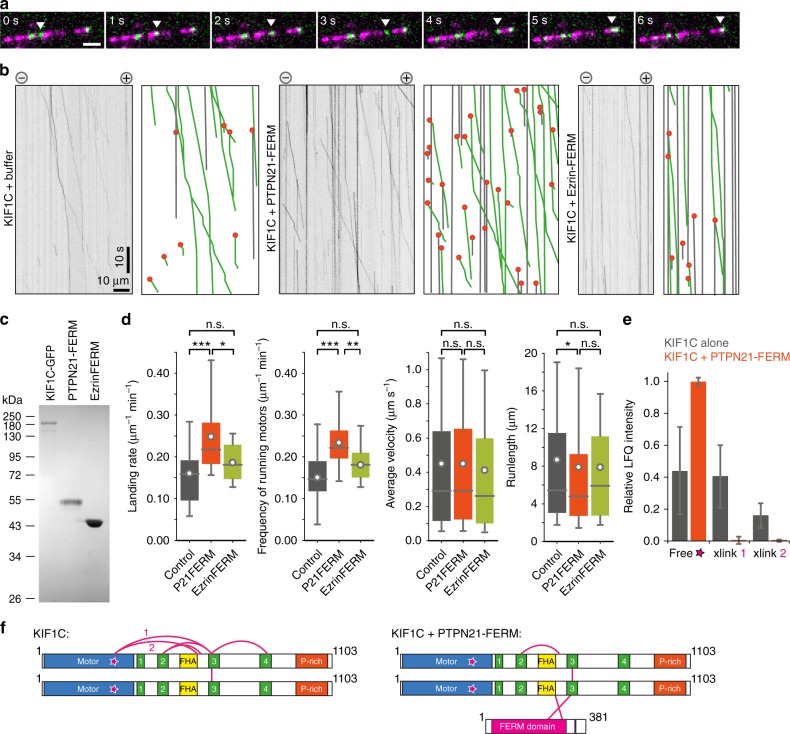
Fig. 5.
PTPN21 FERM domain activates KIF1C in vitro. a KIF1C-GFP (green) is a processive motor in single-molecule assays on Taxol-stabilised microtubules (magenta). Scale bar 2 µm. b Representative kymographs from single-molecule experiments of KIF1C in the presence of FERM domains of PTPN21 and Ezrin. Grey lines indicate immobile motors; green lines running motors and orange dots landing events. c Coomassie-stained SDS-PAGE of purified KIF1C-GFP and FERM domains of PTPN21 and Ezrin. d Quantification of landing rate, frequency of running motors (>25 nm/s), average velocity and run length. n = 30, 39 and 21 microtubules respectively from three independent experiments. *p < 0.05, **p < 0.005, ***p < 0.0005, n.s. p > 0.05 (t-test with Bonferroni correction). Data are provided in Source Data file. e, f Crosslinking mass spectrometry of KIF1C in the presence of PTPN21-FERM identified two crosslinks between KIF1C and the FERM domain. Crosslinks in KIF1C alone are shown for comparison. Star indicates peptide in motor domain that is found in crosslinks with stalk domain (labelled 1 and 2). Relative abundance of single peptide LKEGANINK (star) and the two crosslinked peptides between motor and stalk domain are shown for KIF1C alone and KIF1C + PTPN21-FERM. Data show mean ± SD for three independent experiments. See Supplementary Figs. 2, 3 and 5 for ion fragmentation spectra. Data are provided in Source Data file