Abstract
Objective
To report the results of the posterior approach for thoracic ossification of posterior longitudinal ligament (TOPLL) by using a special “L” osteotome.
Methods
The present study enrolled 16 consecutive patients (9 men and 7 women) between May 2009 and September 2013. All patients underwent a posterior circumferential decompression osteotomy and segmental instrumentation with interbody fusion. The mean age at surgery was 57.3 years (range, 37–68 years). Patients’ data, clinical manifestation, blood loss, length of surgery, complications, visual analog scale (VAS), Japanese Orthopedic Association (JOA), and Frankel grading system before and after surgery were collected and evaluated, retrospectively.
Results
The average follow‐up period was 30 ± 19 months (range, 12–50 months). All patients were successfully treated with posterior compression and segmental instrumentation with interbody fusion. The average operation time was 261.6 ± 51.3 min (range, 190–310 min). The mean blood loss was 980.3 ± 370.5 mL (range, 600–2100 mL). All patients had subjective improvement of motor power and gait. Average preoperative and postoperative JOA scores were 4.2 ± 1.7 and 7.8 ± 2.5 points, respectively. Differences in the overall JOA scores showed significant postoperative improvement. At the last follow‐up, all patients improved either by one or two Frankel grades. There was a significant difference between preoperative VAS scores and those 3 months after surgery (P < 0.05). No significant difference was observed between the 3‐month and 12‐month results (P > 0.05). Cerebrospinal fluid (CSF) leakage occurred in 3 patients. Acute neurological deterioration was encountered postoperatively in 1 patient.
Conclusion
Treatment with posterior transpedicular osteotomy and circumferential decompression was found to be safe, effective, reliable, and technically feasible, and keeping the thoracic cavity intact avoids many shortcomings of anterior surgery and results in a satisfactory spinal decompression.
Keywords: Circumferential decompression, Internal fixation, Ossification of the posterior longitudinal ligament, Osteotomy
Introduction
Thoracic ossification of the posterior longitudinal ligament (T‐OPLL) is the heterotopic ossification of the spinal ligament; it has an unclear pathogenesis. Ohtsuka reported a T‐OPLL incidence of approximately 0.8%1. It is a rare disease, while its concomitant spinal cord compression often leads to severe neurological dysfunction, for which conservative treatment is nonexistent and surgery is the only therapeutic method1. To date, multiple surgical procedures have been developed to treat T‐OPLL, including a sternum‐splitting approach, anterior decompression through a lateral subscapular approach, anterolateral decompressive transversectomy2, posterior decompressive laminectomy or laminoplasty, circumferential decompression through a posterior approach, posterior spinal decompression, bone grafting and internal fixation, and posterior and anterior decompression2, 3, 4, 5, 6, 7, 8, 9, 10, 11. Each procedure has respective advantages and disadvantages. A multicenter study of T‐OPLL surgical therapy hosted by Matsumoto et al. demonstrated that although each surgical procedure can achieve a certain clinical efficacy, T‐OPLL presented a higher incidence of surgical complications12. Furthermore, the pros and cons of both the complications and clinical efficacy incidence between different surgical approaches, as well as the most optimal surgical approaches, remain controversial. There is a lack of unified standards for surgical approach selection4, 10. Thus, there is a need to establish adequate protocols for the treatment of thoracic T‐OPLL13.
Anterior decompression is the most ideal surgical procedure, because it can achieve direct dissection of the ossified posterior longitudinal ligament and achieve complete spinal cord decompression8. However, it has a limited clinical application due to large trauma, interference with pulmonary function, the complex surgical procedure, and a high incidence of postoperative complications4, 14, 15, 16. Posterior decompression is relatively simple, with fewer complications17. However, due to thoracic kyphosis, which limits the posterior shift of the spinal cord, it is unlikely to achieve total resection of the ossified ligament; it immediately risks aggravating postoperative spinal cord injury, and has unsatisfactory long‐term efficacy.
In recent years, many researchers have proposed adopting a first‐phase articular process surgery through the posterior approach, in order to achieve thoracic spinal cord circumferential decompression9. This method integrates advantages of both anterior and posterior approaches. It is theoretically feasible; however, the ossified segments cannot be directly operated on due to the limited operational vision. Repeated retraction of the spinal cord may increase the risk of spinal cord injury, resulting in greater operating difficulty and a higher incidence of complications. Furthermore, few reports exist on first‐phase posterior circumferential decompression osteotomy and selection of the osteotomy boundary; there is a lack of corresponding operational standards.
Therefore, we improved the circumferential decompression through a posterior approach; a new osteotomy (transpedicular osteotomy) was designed. The objective of the current study was to report the outcomes of a posterior approach in 16 patients with T‐OPPL using a novel designed L‐shaped osteotome and to evaluate its efficacy and feasibility.
Clinical Materials and Methods
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (i) typical spinal cord compression symptoms; (ii) continuous or noncontiguous T‐OPLL with/without ossification of ligamentum flavum; and (iii) severe chest back pain, which was not significantly improved after conservative treatment for 6 months.
Exclusion criteria were as follows: (i) aymptomatic T‐OPLL; (ii) calcified thoracic disc herniation; and (iii) unable to tolerate the operation due to poor condition.
Patient Population
Between May 2009 and September 2013, a total of 16 T‐OPPL patients (9 men and 7 women) who received a posterior pedicle osteotomy, circumferential decompression, and internal fixation were selected, including 10 patients with continuous T‐OPLL and 6 patients with noncontiguous T‐OPLL. Their ages ranged from 37 to 68 years, with a mean of 57.3 years. The location of lesions included three cases of the upper thoracic spine (T1–T4), four cases of the mid‐thoracic spine (T5–T8), and nine cases of lower thoracic spine (T9–T12). Of these, ossification of the ligamentum flavum was found in two cases.
Clinical Manifestation
All the patients presented with typical spinal cord compression symptoms, including chest and back pain, zonesthesia in the thoracic and abdominal regions, significant sensory disturbances, numbness in the saddle area and lower extremities, lower extremity weakness, muscular tension in the lower limbs, reflex activity or hyperreflexia of the knees and ankles, patellar clonus, ankle clonus, and a positive Babinski sign. A total of 4 patients had accompanying intercostal neuralgia, 7 patients had sphincter dysfunction, and 3 patients demonstrated muscle atrophy. All the patients received X‐ray films on the lateral side of the lesions, reconstructive images using CT plain sagittal views, as well as MRI examinations. X‐ray films were used for body positioning; CT images were used to clarify the morphology and scope of T‐OPLL to determine both the osteotomy scope and the ossification of the dural sac. High‐density ossification of the posterior edge of the vertebral body was seen on CT images. MRI results were used to reveal both the amount of spinal cord compression due to OPLL and the accompanying spinal cord degeneration, which showed low signal in the T1 and T2 weighted image.
Osteotomy Scope and Boundary
Spinal stenosis segments and the respective compressed spinal cord segments were determined using a preoperative MRI examination. The “triangular safe area” was identified in the MRI midsagittal T2‐weighted images18. As the dural sac was compressed by the OPLL, there was a potential triangular space at the OPLL ends, the posterior edge of the vertebral body, and the compressed dural sac, known as the “triangular safe area”. In this area, the compressing mass could be safely removed using a bone knife (Fig. 1). This “triangular safe area” was considered to be the beginning‐point of the “L” osteotomy. A preoperative reconstruction using CT plain sagittal view scans was performed to determine the length and types of the ossified posterior longitudinal ligament; this identified the positional relationship between the OPLL ends and pedicle, clarifying the osteotomy scope.
Figure 1.
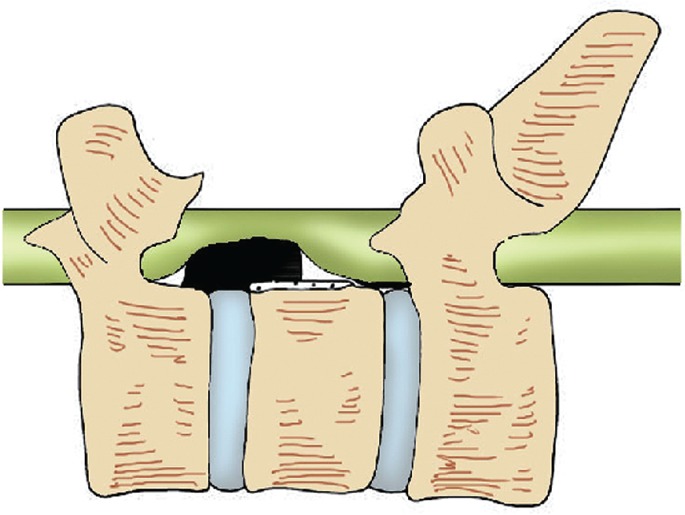
Diagram of the “triangular safe area,” where normal and ossifying posterior longitudinal ligament is cut off. As the dural sac was compressed by the ossification of the posterior longitudinal ligament (OPLL), there was a potential triangular space at the OPLL ends, the posterior edge of the vertebral body, and the compressed dural sac, known as the “triangular safe area.”
The osteotomy boundary at the head and tail ends of OPLL was determined by axial and sagittal view of CT. On axial CT view, the invasion of the OPLL to the spinal transverse diameter and the pedicular inner wall involvement at the corresponding levels were identified. When the unilateral or bilateral pedicular inner walls at the corresponding levels involved the OPLL, the “triangular safe area” was not fully exposed due to the pedicle blockage. Hence, removal of the unilateral or bilateral pedicles at the corresponding levels was necessary to expose the “triangular safe area”. On the CT sagittal view, when the OPLL head and tail ends were not completely blocked by the pedicle, the pedicle was retained and screw placement occurred when the “triangular safe area” could be exposed. When the “triangular safe area” was completely blocked by the pedicle, we needed to either partially or completely remove the pedicle to fully expose the “triangular safe area”; the screw placement was performed in the superior (inferior) pedicle.
The horizontal level of OPLL would not be more than the transverse diameter of the thoracic spinal canal (bilateral intrapedicular diameter). Thus, the medial walls of the bilateral pedicles were considered the bilateral osteotomy boundary (Fig. 2).
Figure 2.
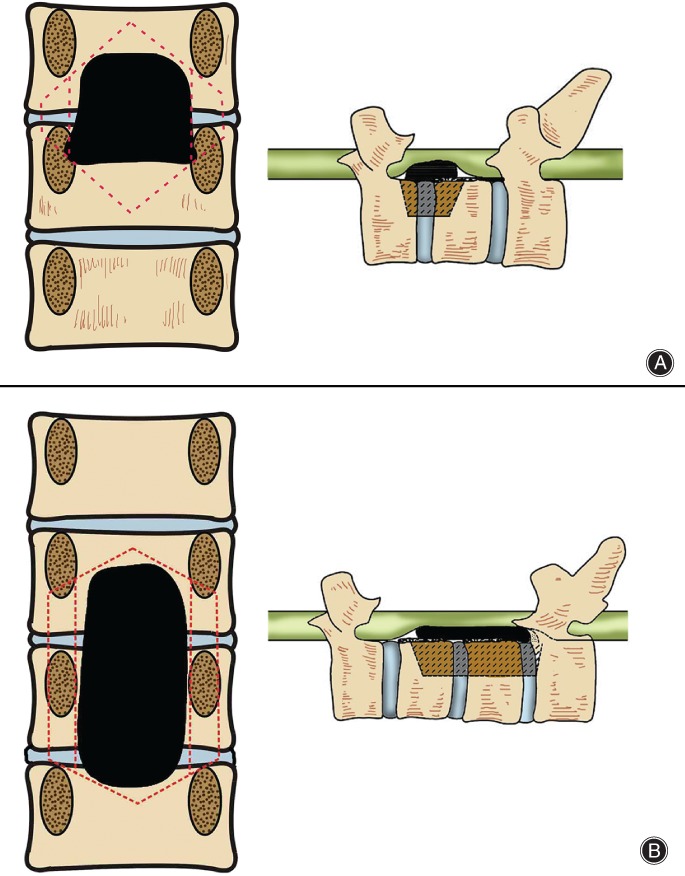
Osteotomy scope in coronal and sagittal views of thoracic ossification of posterior longitudinal ligament (TOPLL). (A) Head end of OPLL is in middle–lower part of pedicle and the “triangular safe area” is not completely blocked; the pedicle can be retained in osteotomy. Invasion of the tail end of the OPLL to the transverse diameter of the spinal canal is 100%, and involves the bilateral pedicle inner wall. The osteotomy requires removing the corresponding pedicle. The exterior and inner walls of the pedicle are the exterior and inner boundaries of the osteotomy. (B) Head end of OPLL is in the middle–upper part of the pedicle, and the “triangular safe area” is blocked by the pedicle. Thus, the procedure requires partial or total pedicle removal to expose the “triangular safe area”. Resection of pedicle in the middle area of OPLL is necessary. The tail end of the OPLL is in the middle–upper part of the pedicle. The “triangular safe area” is not completely blocked, and the pedicle can be retained as part of the osteotomy procedure.
Surgical Technique
Patients were placed in the prone position after endotracheal intubation and general anesthesia. The surgical levels were determined using a C‐arm X‐ray machine; a median incision was then performed to expose the spine and the periosteum was stripped to expose the acantha, neural plate, articular processes, and costotransverse articulations that were beyond two spinal levels of the decompressed levels. The transverse processes were removed first, followed by the posterior wall of the spinal canal, using a marsupialization method. A longitudinal groove was opened at the midline of the articular process (the pedicle transverse section was close to its inner edge) using a high‐speed drill. The upper edge of the head or tail end of the neural plate within the decompressed range was grained down and lifted, at which the entire posterior wall of the spinal canal was lifted at the same time; the adhering zone below the neural plate was isolated, and the posterior wall of the spinal canal was removed like a “take‐off lid”, allowing for its decompression. After an incision was made into the posterior wall of the spinal canal, the intervertebral nerve root was exposed and protected; the bilateral residual articular process was completely removed using a high‐speed drill or bone rongeur. The pedicle was resected until the posterior edge of the vertebral body to achieve more operative space to directly reveal the ventral spine. The posterolateral intervertebral discs in the incision range were revealed first. The fibrous rings at the posterolateral intervertebral disc were routinely removed; tissue located within intervertebral disc tissue was removed to release the intervertebral space.
After that step, a 30° oblique longitudinal groove from the bilateral posterior margin to approximately half of the vertebral body was made using a high‐speed drill, curette, and bone knife going along the axial direction of the pedicle from the bilateral exterior margin of the pedicle. A 60° oblique longitudinal groove was made from the bilateral internal margins of the pedicle and posterior margin of the vertebral body to one‐quarter of the posterior vertebral body. The vertebral cancellous bone between the two grooves was then dug out, and an intravertebral “culvert” was formed in the frontal OPLL after the bilateral bone troughs were opened up. A nerve dissector was then used to carefully explore both the head and tail ends of the OPLL in the ventral spinal cord to reveal the “triangular safe area”, and hemostasis was performed. An “L” model osteotome was placed in the “triangular safe area” between the head and tail ends of OPLL, and adjacent to the ventral dural sac midline; at the non‐ossified area of the posterior longitudinal ligament, the OPLL was cut off from the non‐ossified posterior longitudinal ligament. The OPLL was isolated from the normal posterior longitudinal ligament after realignment. The adhering zone between the dural sac and the compressing mass was isolated using a nerve dissector; meanwhile, the compressing mass was pushed into the “culvert”. During this time, part of the dural sac was removed if necessary. Then, the OPLL mass was removed from the bilateral bone troughs using a pith nucleus clamp, completing the decompression of the frontal spinal cord. After that, the pedicular screw was placed and the connective bar was assembled. Intervertebral pressure was applied to improve the bone grafting rate while correcting kyphosis (Figs 3, 4, 5). After hemostasis, the drainage tube was placed and then the wound was closed.
Figure 3.
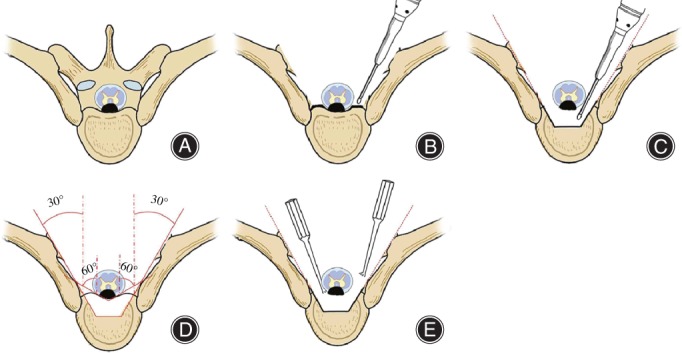
Schematic diagram of thoracic transpedicle osteotomy and circumferential decompression. (A) Diagram of thoracic ossification of posterior longitudinal ligament (TOPLL); (B) both lamina and bilateral articular processes are removed and the bilateral pedicles are abraded to the posterior edge of the vertebral body. The costotransverse articulations are retained, while the dural sac, nerve root, and “triangular safe area” are exposed; (C) transpedicle osteotomy is performed, where the shadow shows the resected part; (D) a “culvert” is generated after resection of one‐quarter to half of the posterior vertebral body. The OPLL is floating above the culvert; and (E) the OPLL is cut off using an “L” model osteotome and pushed into the “culvert”.
Figure 4.
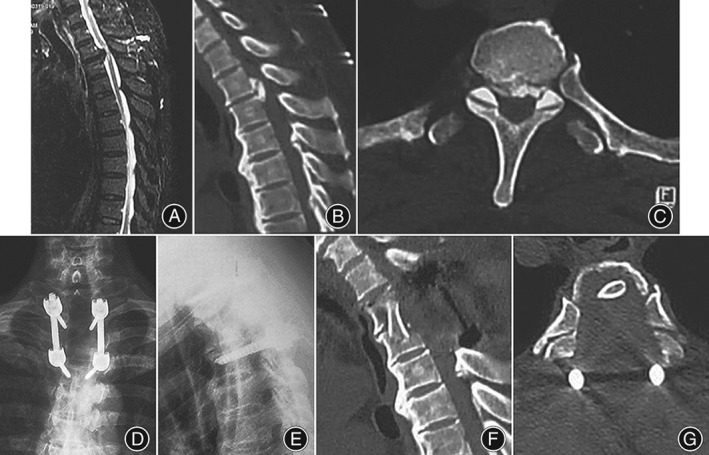
Imaging of the preoperative and postoperative surgery levels (female, 57 years old). (A) Preoperative MRI shows a unisegmental bird‐beak thoracic ossification of posterior longitudinal ligament (TOPLL), with significant compression at the T 2–3 spinal cord plane; (B) preoperative sagittal CT scan shows a unisegmental isolated T‐OPLL at T 2–3; and (C) preoperative axial CT scan shows the tail end of OPLL is at the T 3 pedicle. The invasion of the OPLL to the transverse diameter of the spinal canal is 100%, involving the inner wall of the bilateral pedicle; (D, E) postoperative lateral side X‐ray photograph shows pedicle screw internal fixation at T 2 and T 4, with a good fixation position; and (F) postoperative sagittal CT scan shows the “culvert” has been removed. (G) A postoperative axial CT scan shows total removal of the T 3 OPLL; complete decompression of spinal cord is achieved, and costotransverse articulations were retained.
Figure 5.
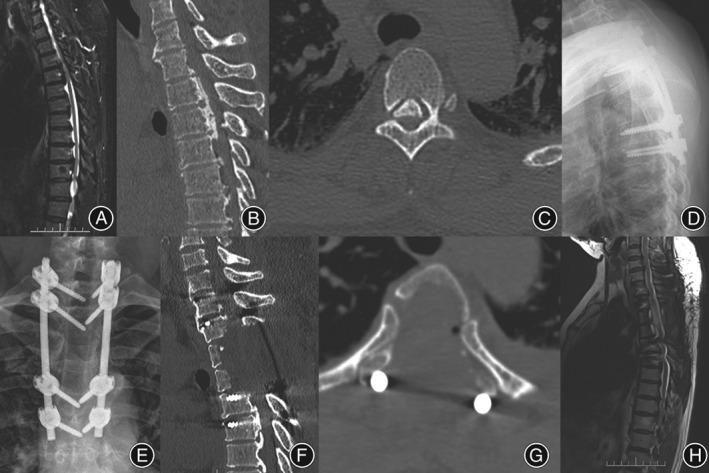
Imaging of the preoperative and postoperative surgery levels (male, 46 years old). (A) Preoperative sagittal T 2‐weighted MRI shows a mixed T‐OPLL, with significant compression in spinal cord planes of T 2–3, T 3–4, and T 5–6; (B) preoperative sagittal CT scan shows continuous OPLL in planes of T 2–3, T 3–4, and T 5–6; (C) preoperative axial CT scan reveals that the OPLL tail end is at the T 5 pedicle level, the occupation ratio of the OPLL on the transverse diameter of spinal canal is less than 100%, and the bilateral pedicular inner wall is not involved; (D, E) postoperative lateral side X‐ray photography reveals pedicular screw internal fixation at T 1, T 2, T 4, and T 5 levels, with a good fixation position; (F) postoperative sagittal CT scan shows complete resection of the OPLL; (G) postoperative axial CT scan shows a total resection of OPLL at T 3, with a complete decompression of the spinal cord, while retaining the costovertebral joints; (H) a postoperative sagittal T 2‐weighted MRI shows the total resection of the OPLL and complete decompression of the spinal cord.
Evaluation and Follow‐up
The patients were reviewed at 3 months after surgery, followed by 3‐month intervals for the remainder of the first 1 year after time of surgery with subsequent follow‐up every 1 year, until the last follow‐up. The visual analog scale (VAS) was used to measure changes in pain from preoperative to postoperative levels. Preoperative and postoperative symptoms were assessed by Frankel neurological grade and Japanese Orthopedic Association (JOA) score for thoracic myelopathy10, 11. The recovery rate was calculated using the Hirabayashi method: (postoperative JOA score – preoperative JOA score)/(11 − preoperative JOA score) × 100%19. A recovery rate of >75% was graded as excellent, 50%–74% as good, 25%–50% as fair, and <25% as poor.
Statistical Analysis
The data were presented as mean ± standard deviation (SD) and analyzed using SPSS for Windows, version 16.0 (SPSS, Chicago, IL, USA). Repeat analysis of variance measurements were used to assess statistical significance of both preoperative and postoperative parameter changes. A P‐value of <0.05 was considered statistically significant.
Results
Clinical Outcomes
The mean operation time was 261.6 ± 51.3 min, and ranged from 190 to 310 min. The average blood loss was 980.3 ± 370.5 mL (range, 600–2100 mL) and the period of postoperative immobility was 6.8 ± 4.5 days (range, 3–14 days). The follow‐up period was 31.5 ± 6.7 months (range, 26–47 months). In all patients, a complete removal of the calcified disc was ascertained by a postoperative CT scan.
Functional Results
Visual Analog Scale Score
The mean preoperative VAS score was 8.01 in all patients, which improved to 2.70 points at 3 months, and 2.37 at 1 year; this further decreased to 2.01 at the last follow‐up in 14 cases. Statistical analysis of the results showed a significant improvement of pain at 3 months (P < 0.05) when compared to the preoperative status. No significant difference was observed between the 3‐month and 12‐month results (P > 0.05).
Japanese Orthopedic Association Score
The JOA score was used to quantitatively evaluate postoperative neurological improvement. In all patients, the total JOA score increased after surgery. The mean preoperative JOA scale score was 4.2 ± 1.7 in the series. After surgery, the mean JOA scale score improved to 7.8 ± 2.5 at 3 months and to 8.5 ± 2.7 at the final follow‐up, representing a significant increase as compared with the preoperative score (P < 0.01). Meanwhile, the mean recovery rate at the final follow‐up was 72% ± 8%. The overall results were excellent in 8 patients, good in 5 patients, and fair in 3 patients. No patient was classified as having either unchanged or worse results.
Frankel Grading
The patient neurological status was classified using the Frankel grading system. Four patients were classified as Grade B, 9 patients as Grade C, and 3 patients as Grade D. At the last follow‐up, 16 patients improved either by one or two grades; 1 patient improved from Grade B to C, 3 patients improved from Grade B to D, and 7 patients improved from Grade C to E.
Among the 4 patients with intercostal neuralgia, the symptom disappeared in 3 cases after the surgery, and 1 case appeared to be transient pain aggravation. We suspected that transient pain was caused by interoperative retraction of the nerve root. The patient achieved significant alleviation after receiving neurotrophic hormone and other drug therapy for a period of 3 days; the symptom disappeared 1 week later. Among the 7 patients with sphincter dysfunction, 6 patients were restored to normal at 1‐year follow‐up; 1 patient still appeared to be suffering from urine retention, although in smaller levels as compared with preoperatively.
Complications
Intraoperative cerebrospinal fluid (CSF) leakage occurred in 3 patients. Two cases were caused by adhesion between the dural sac and OPLL; the other cases were induced by dura mater ossification. We sutured two leakage sites with 6 to 0 braided silk and repaired the other with a Neuro‐Patch (Braun Aesculap AG & Co KG, Tuttlingen, Germany). Wound infection occurred in 1 patient at 7 days after surgery; this patient was cured after taking antibiotics based on sensitivity testing at 20 days after operation.
Acute neurological deterioration was encountered postoperatively in 1 patient. Methylprednisolone sodium succinate and ganglioside were administered intravenously; muscle strength gradually improved at 1 week postoperatively. The patient achieved ambulation with the aid of a walker at final follow‐up.
Discussion
The main purpose of T‐OPLL surgical therapy is to remove the ossified lesions and decompress the spinal canal. The ideal method is to directly remove the ossified lesion in the ligament. However, as the dura mater in the ventral spine and the dorsal subarachnoid space are relatively fragile components, any slight retraction or compression may lead to catastrophic consequences related to the spinal cord. Therefore, any surgical treatment of the T‐OPLL is challenging.
Advantages and Disadvantages of Traditional Treatment
Many authors have reported on extensive posterior decompression, expecting indirect decompression resulting from a posterior shift of the spinal cord6, 20, 21, 22. Yamazaki et al. found that posterior decompression alone is unlikely to significantly improve nerve function, particularly when spinal reconstruction stability is poor; consequently, the probability of spinal cord injury increases along with thoracic kyphosis, possibly resulting in short‐term and long‐term postoperative neurological deterioration along with an increased risk of paralysis10. Yamazaki et al. also report that neurological deterioration might appear after posterior laminotomy decompression; in the second phase, stable neurological function may improve after fixation and reconstruction of the spine23. Some authors have tried to prevent postoperative kyphosis through either laminoplasty or fusion with bone grafting supported by instrumentation6, 20, 21. However, these procedures have not always shown satisfactory results, because the OPLL plaque remains and may partially compress the spinal cord due to limitations associated with a posterior shift of the spinal cord6, 20.
The surgical procedure for posterior decompression is relatively simple, with fewer complications than with other procedures. Meanwhile, the wide application of internal fixation instrumentation has increased spinal stability after posterior decompression. In most patients, this is accompanied by ossification of the ligamentum flavum (OLF), and posterior laminotomy decompression is performed; bone grafting and internal fixation has become a compromised surgical method17. Anterior decompression is the most ideal surgical procedure, because it can directly remove the ossified posterior longitudinal ligament and achieve complete spinal cord decompression. The sternotomy approach is mostly applied to the upper thoracic spine, while a corpectomy through the extrapleural or extraperitoneal approach is applied to both the middle and lower thoracic spine8, 20, 22. Diverse approaches exist for the anterior decompression of the thoracic OPLL, and all can achieve complete decompression. However, the complex surgical anatomy may lead to injury, difficulty of operation, and a high incidence of postoperative complications4, 12, 14, 16, including neurological deterioration, epidural hematoma, cerebrospinal fluid leakage, pleural effusion, pneumonia, and nerve root irritation. Fujimura et al. believed that anterior surgery might have relatively good efficacy in treating limited OPLL at the middle and lower thoracic vertebrae. However, a high risk exists of intraoperative spinal cord injury, thereby inducing epidural damage, which is difficult to repair. The incidence of cerebrospinal fluid leakage was 13.3% in this group24. Yamazaki et al. also believed that anterior resection of the T‐OPLL may achieve good efficacy, but has a high intraoperative risk, with a spinal cord injury risk of as much as 18.8%10. Hanai et al. report on T‐OPLL incision surgery via the anterior approach, where 2 out of 12 cases appeared to have spinal cord injuries. All of these results suggested that anterior surgery has a high risk and multiple complications, thereby requiring a more intricate surgical technique. In addition, OPLL is often accompanied by thoracic OLF; anterior decompression is not able to completely relieve OLF‐associated compression4.
Anterior or posterior decompression alone is not able to achieve the ideal decompression results. Thus, we were required to consider a combination of anterior and posterior circumferential decompression techniques17. First, we relieved spinal cord compression through a posterior approach, and then performed the anterior decompression. Tomita et al. suggest performing a posterior laminotomy decompression and internal fixation in the first phase, and conducting anterior decompression to remove the ossified posterior longitudinal ligament in the second phase22. They performed circumferential decompression in two phases and achieved satisfactory clinical efficacy21. In the anterior–posterior operation, the width and resection range of the ossified posterior longitudinal ligament are identified using a posterior approach, which effectively avoids blind stripping due to the failure to directly expose the posterior longitudinal ligament using the anterior approach alone. However, scholars have various opinions on anterior decompression timing in the second phase after the posterior decompression in the first phase. Kawahara et al. suggest conducting an MRI review 3 weeks after the first‐phase decompression, and suggest that as long as spinal compression is seen in the MRI exam, it is appropriate to perform second‐phase anterior decompression regardless of the improvement of symptoms8. Matsuyama et al. believe that patients with an unsatisfactory recovery 6 months after the first‐phase decompression should receive second‐phase anterior surgery11.
With the development of surgical techniques, many researchers have proposed that in the first‐phase posterior circumferential decompression, we should first perform posterior laminotomy decompression to release a space for the posterior shift of the spinal cord. This action would greatly reduce the spinal cord injury risk during resection of the anterior compressing mass; the lateral articular process or costovertebral joint were removed to the site of the lesion, reducing the risk of paralysis caused by an intraoperative dural sac retraction. This method integrates advantages of anterior and posterior approaches, which is feasible in theory. However, in practice, this operational approach has limitations, as failing to perform an operation of the ossified segments under direct vision can result in an increased spinal cord injury risk due to intraoperative repeated retraction of the spinal cord. The surgery is difficult and has a high incidence of complications; it is not easy to correct segmental kyphosis. According to Takahata et al. the first‐phase posterior circumferential decompression might offer a good prognosis in patients with short‐segment disease, while multilevel decompression may induce various complications. The respective incidence of postoperative neurological deterioration, dural laceration, and deep infection were 33.3%, 40%, and 10%9. Yang et al. report cases receiving costotransverse resection and circumferential decompression through a posterolateral approach; they believe that this approach is appropriate for resection of a single huge thoracic OPLL2.
Modified Transpedicular Approach
A transpedicular approach is more appropriate for spine surgeons. It removes bilateral pedicles in order to achieve more operational space and perform the ventral spinal operation under direct vision. Preoperative MRI examination was performed on lesions to determine the responsible compressed segments of the spinal cord. In addition, a CT plain scan was performed to determine the relationship between OPLL and the pedicle correspondingly at different levels to identify the scope of the osteotomy. During the surgery, marsupialization was adopted to remove the posterior wall of the spinal canal, thereby achieving decompression of the posterior spinal canal. A high‐speed drill was then used to grind the bilateral articular processes; the pedicle was cut to the level of the posterior vertebral margin to achieve greater operative space and to carry out ventral spinal operation under direct vision. Along the pedicular axial direction at the bilateral exterior and inner margins, a high speed drill and curette made oblique 30° and 60° longitudinal grooves at the vertebral posterior margin; the cancellous bone between the two grooves was dug out, and an intravertebral “culvert” was generated in the frontal OPLL after the bilateral bone troughs were opened up. An “L” model osteotomy was placed in the “triangular safe area” at two ends of the OPLL; the ossified posterior longitudinal ligament was cut off and pushed forward to the “culvert” and removed, thus completing the decompression in the frontal spinal cord.
Precautions of Transpedicular Approach
There were several intraoperative precautions. This surgical approach required resection of the middle and posterior spinal column of the decompressed segments, significantly influencing the spinal stability. Such an approach is needed to conduct pedicle screw fixation, and a correction can be simultaneously carried out to reduce the incidence of postoperative kyphosis8, 11. Interbody bone grafting is required for resection cases involving more than half of the vertebral body and to avoid damage to the corresponding nerve roots during the resection of articular process. Sharp objects and electric coagulation are required to avoid the nerve root located at the lower margin of the upper pedicle.
Posterior circumferential decompression was prone to cause bleeding in two areas. One area is the posterior segmental vascular hemorrhage after resection of the articular process. In most cases, bipolar electric coagulation is used, or combined with cotton slices or compression hemostasis with a gel sponge. Another area at risk of bleeding is the epidural venous plexus in the resection of ventral compressing mass, usually when dural film and compression hemostasis with a gel sponge are applied. Special attention must be paid to postoperative unobstructed drainage and remission of lower limb motor function. Emergent hematoma aspiration shall be performed once an epidural hematoma occurs.
During the osteotomy operation, the “triangular safe area” is first revealed, and bilateral removal of the intervertebral disc is performed to release the intervertebral space. Next, an “L” model osteotome is placed in the “triangular safe area”, which is adjacent to the ventral dural sac midline. The OPLL is cut off from the normal posterior longitudinal ligament in the non‐ossified normal area of the posterior longitudinal ligament; it is isolated from the normal posterior longitudinal ligament after bilateral realignment. The most difficult operational procedure in the posterior circumferential decompression involves the OPLL resection in the ventral spinal cord. A transpedicular osteotomy is first performed to obtain a “culvert”, and then the compressing mass is pushed to the “culvert”; the OPLL resection requires applying force forward along the paths “departing” the spinal cord, which is a key technique to ensure the safety of the spinal cord. The indications for this surgical procedure are short segment length, limited OPLL, and intervertebral disc calcification; relative contraindications include the presence of a long‐segmental and continuous thoracic OPLL involving more than four vertebral segments.
Advantages of Transpedicular Approach
Advantages of the OPLL technique include those associated with a traditional posterior approach. It is a familiar surgical approach for spine surgeons and, thus, may induce less surgical stress; the procedure may be useful for a wider range of lesions, as it is easier to control CSF leakage and achieve total resection of the calcified posterior longitudinal ligament. As compared with the traditional technique, the bilateral pedicle is removed to provide a broader vision; this enables all the operations to be performed under direct vision, thereby ensuring the safety of the operation. This technique does not require entering the thoracic cavity, consequently reducing the incidence of pulmonary complications. In regards to upper thoracic spine surgery, this approach is safer than the anterior surgical approach; in the latter, the mediastinum can be impacted, leading to difficulty and dangers associated with this region. The traditional surgical procedure only removes the OPLL in the lower thoracic and thoracolumbar spine. In comparison, the transpedicular spinal circumferential decompression can be applied to OPLL patients, and involves the removal of all segments from the upper thoracic spine to the thoracolumbar spine. A combination of thoracic OPLL and OLF can achieve complete decompression of ventral and dorsal vertebrae. For diseased segments with a larger local kyphotic angle, we can perform pedicle screw internal fixation and bone grafting to reconstruct spinal stability, applying intervertebral pressure to improve the bone grafting rate and correct a kyphotic deformity indirectly effecting spinal decompression.
Conclusion
Thoracic ossification of posterior longitudinal ligament treatment with posterior transpedicular osteotomy and circumferential decompression is safe, effective, reliable, and technically feasible. Keeping the thoracic cavity intact avoids many shortcomings of anterior surgery, and results in a satisfactory spinal decompression. Combining this approach with pedicular screw internal fixation helps to reconstruct spinal stability, correct kyphosis, and achieve satisfactory clinical results.
Acknowledgments
The authors would like to thank Jing sun who helped to polish this manuscript and the reviewers and editors of Orthopaedic Surgery for their review and constructive criticism in improving the manuscript.
Disclosure: No funds were received in support of this work.
References
- 1. Matsumoto M, Chiba K, Toyama Y, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine: a multi‐institutional retrospective study. Spine (Phila Pa 1976), 2008, 33: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 2. Yang C, Bi Z, Fu C, Zhang Z. A modified decompression surgery for thoracic myelopathy caused by ossification of posterior longitudinal ligament: a case report and literature review. Spine (Phila Pa 1976), 2010, 35: E609–E613. [DOI] [PubMed] [Google Scholar]
- 3. Kojima T, Waga S, Kubo Y, Matsubara T. Surgical treatment of ossification of the posterior longitudinal ligament in the thoracic spine. Neurosurgery, 1994, 34: 854–858. [DOI] [PubMed] [Google Scholar]
- 4. Hanai K, Ogikubo O, Miyashita T. Anterior decompression for myelopathy resulting from thoracic ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976), 2002, 27: 1070–1076. [DOI] [PubMed] [Google Scholar]
- 5. Tsuzuki N, Hirabayashi S, Abe R, Saiki K. Staged spinal cord decompression through posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976), 2001, 26: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 6. Tokuhashi Y, Matsuzaki H, Oda H, Uei H. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification‐kyphosis angle on MRI. Spine (Phila Pa 1976), 2006, 31: E26–E30. [DOI] [PubMed] [Google Scholar]
- 7. Aizawa T, Sato T, Sasaki H, et al. Results of surgical treatment for thoracic myelopathy: minimum 2‐year follow‐up study in 132 patients. J Neurosurg Spine, 2007, 7: 13–20. [DOI] [PubMed] [Google Scholar]
- 8. Kawahara N, Tomita K, Murakami H, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976), 2008, 33: 39–46. [DOI] [PubMed] [Google Scholar]
- 9. Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976), 2008, 33: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 10. Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine(Phila Pa 1976), 2006, 31: 1452–1460. [DOI] [PubMed] [Google Scholar]
- 11. Matsuyama Y, Sakai Y, Katayama Y, et al. Indirect posterior decompression with corrective fusion for ossification of the posterior longitudinal ligament of the thoracic spine: is it possible to predict the surgical results?. Eur Spine J, 2009, 18: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto M, Toyama Y, Chikuda H, et al. Outcomes of fusion surgery for ossification of the posterior longitudinal ligament of the thoracic spine: a multicenter retrospective survey: clinical article. J Neurosurg Spine, 2011, 15: 380–385. [DOI] [PubMed] [Google Scholar]
- 13. Seichi A, Takeshita K, Nakahara K. Choice of surgical procedure for thoracic ossification of the posterior longitudinal ligament In: Yonenobu K, Nakahara K, Toyama Y, eds. OPLL Ossification of Posterior Longitudinal Ligament, 2nd edn. Tokyo: Springer, 2006; 225–230. [Google Scholar]
- 14. Yamazaki M. Updates of ossification of posterior longitudinal ligament. Clinical results and complication of surgery for thoracic myelopathy due to ossification of posterior longitudinal ligament. Clin Calcium, 2009, 19: 1499–1504. [PubMed] [Google Scholar]
- 15. Inamasu J, Guiot BH. A review of factors predictive of surgical outcome for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg Spine, 2006, 5: 133–139. [DOI] [PubMed] [Google Scholar]
- 16. Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech, 2008, 21: 116–119. [DOI] [PubMed] [Google Scholar]
- 17. Yamazaki M, Okawa A, Fujiyoshi T, Furuya T, Koda M. Posterior decompression with instrumented fusion for thoracic myelopathy caused by ossification of the posterior longitudinal ligament. Eur Spine J, 2010, 19: 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu N, Chen Z, Qi Q, Li W, Guo Z. Circumspinal decompression and fusion through a posterior midline incision to treat central calcified thoracolumbar disc herniation: a minimal 2‐year follow‐up study with reconstruction CT. Eur Spine J, 2014, 23: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo JJ, Yang HL, Cheung KM, Tang TS, Luk KD. Classification and management of the tandem ossification of the posterior longitudinal ligament and flaval ligament. Chin Med J (Engl), 2009, 122: 219–224. [PubMed] [Google Scholar]
- 20. Matsuyama Y, Yoshihara H, Tsuji T, et al. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: implication of the type of ossification and surgical options. J Spinal Disord Tech, 2005, 18: 492–497. [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki M, Ohkawa A, Moriya H, Mochizuki M. Surgical indication of the posterior decompression and fusion for thoracic myelopathy due to ossification of the posterior longitudinal ligament of the spine according to its clinical result. East Japan J Orthop Traumat, 2004, 16: 81–83. [Google Scholar]
- 22. Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine (Phila Pa 1976), 1990, 15: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 23. Yamazaki M, Koda M, Okawa A, Aiba A. Transient paraparesis after laminectomy for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spinal Cord, 2006, 44: 130–134. [DOI] [PubMed] [Google Scholar]
- 24. Fujimura Y, Nishi Y, Nakamura M, Watanabe M, Matsumoto M. Myelopathy secondary to ossification of the posterior longitudinal ligament of the thoracic spine treated by anterior decompression and bony fusion. Spinal Cord, 1997, 35: 777–784. [DOI] [PubMed] [Google Scholar]


