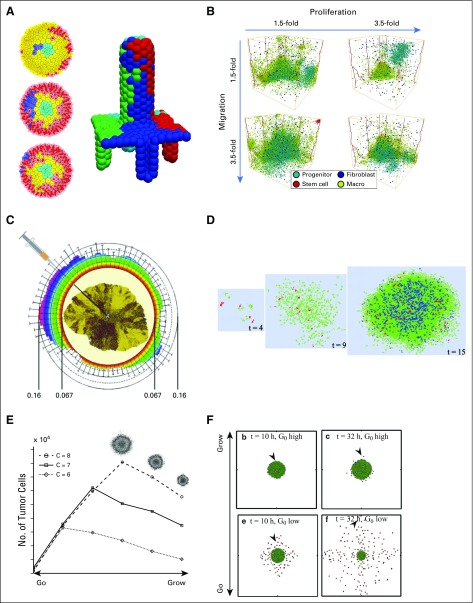
FIG 4.
Cancer stem cells, invasion, and the "go or grow" hypothesis. (A) Top view of a three-dimensional (3D) center-based model of colon crypts (left plots) where the stem-cell niche is in the center. A nonstem mutation (blue cells) is swept out of the crypt by the proliferative cell flux. On the right, is a 3D view of four such ducts that feed cells to a central villus, which is based on the same simulation model. Adapted with permission from Fletcher et al61 (left) and Mirams et al37 (right). (B) A 3D cellular automaton (CA) model (with stem-cell effects) of how chemical signaling with fibroblasts and macrophages can drive triple-negative breast cancer. Among these findings, if stromal cells can promote increased cancer cell migration, the overall tumor grows. Reprinted with permission from Norton et al.62 (C) A 2D CA model to investigate the spread of traits in growing tumors, when cancer cells and their progeny could carry four tumor traits. Traits disseminate largely radially, with clear implications for tumor needle biopsies. Adapted from Poleszczuk and Enderling.11 (D) A 2D cellular Potts model of stem cells in glioblastoma that shows their role in building resistance to radiotherapy. Reprinted with permission from Gao et al.63 (E) Lattice gas CA models of the "go or grow" hypothesis in glioblastoma multiforme. As cells spend more time proliferating, they contribute to better growth up to a critical transition point; beyond this point, decreased migration is insufficient to open space for cell division. Adapted with permission from Hatzikirou et al.64 (F) A center-based model to explore the "go or grow" hypothesis in glioblastoma multiforme. Here, G0 is the models' growth rate parameter. Adapted with permission from Kim et al.65