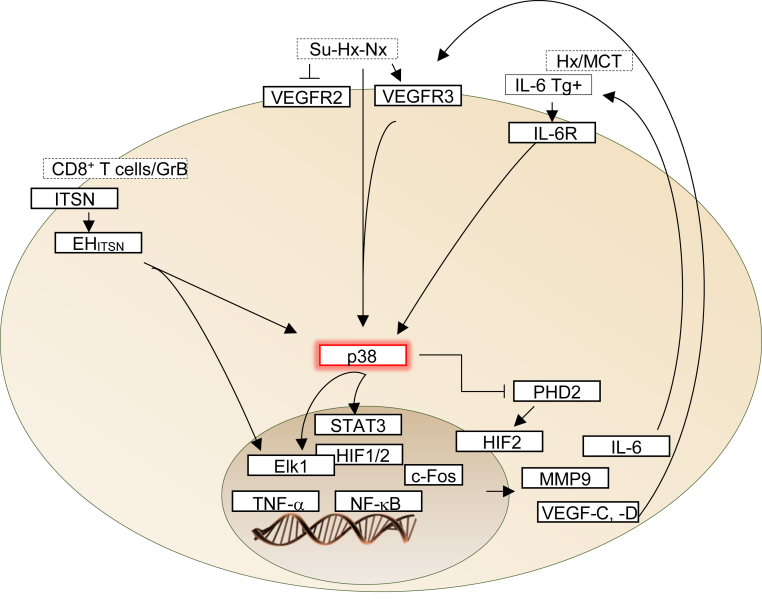
Figure 1.
Signaling pathways that converge on p38 mitogen-activated protein kinase (MAPK) activation and formation of plexiform-like lesions in pulmonary arterial hypertension (PAH). In both hypoxia (Hx) and monocrotaline (MCT) animal models and in human disease, p38 MAPK signaling through IL-6 provides a link between vascular remodeling and inflammation. IL-6 activates STAT3 in a process dependent on serine phosphorylation by p38 kinase and tyrosine phosphorylation by Src. STAT3 activation in both human and experimental models of PAH accounts for the modulation of the expression of several proteins implicated in PAH pathogenesis.61 Protein components of the vascular endothelial growth factor (VEGF) signaling pathway have been implicated as potential biomarkers in experimental models and human PAH; however, the signaling pathways involved are not clear. Interestingly, in cancer cells, a link between VEGF receptor (VEGFR) and p38 kinase has been reported.62, 63 VEGF, acting through its receptor, activates p38 MAPK; and induces expression of erythroblast transformation–specific 1 (ETS1), an important trans regulator of matrix metallopeptidase (MMP) genes, including MMP9. During hypoxia, activation of p38 MAPK signaling is likely to prevent prolyl hydroxylase domain proteins (PHDs) from hydroxylating proline residues of hypoxia-inducible factor (HIF), leading to its activation. Finally, intersectin (ITSN) deficiency and the Epsin15-homology domain fragment of ITSN (EHITSN) expression trigger a pathogenic p38 MAPK/Elk1 signaling pathway, leading to plexiform lesion formation in severe PAH. As EHITSN localizes to the nucleus,64 a direct effect on transcription factors cannot be ruled out. GrB, granzyme B; Su-Hx-Nx, a model in which, after a single injection of Sugen5416 and 3 weeks of hypobaric Hx, the rats were returned to normoxia for 10 to 11 weeks; Tg, transgenic; TNF-α, tumor necrosis factor-α.