Abstract
Background
In patients with suspected cardiac sarcoidosis, late gadolinium enhancement on cardiovascular magnetic resonance imaging and/or 18F‐fluorodeoxyglucose uptake on positron emission tomography are often used to reach a clinical diagnosis of cardiac sarcoidosis. On the basis of data from the imaging literature of clinical cardiac sarcoidosis, no specific features of myocardial involvement are regarded as pathognomonic for cardiac sarcoidosis. Thus, a diagnosis of cardiac sarcoidosis is challenging to make. There has been no systematic analysis of histologically diagnosed cardiac sarcoidosis for patterns of myocardial involvement. We hypothesized that certain patterns of myocardial involvement are more frequent in histologically diagnosed cardiac sarcoidosis.
Methods and Results
We performed a systematic review and meta‐analysis of gross pathological images from the published literature of patients with histologically diagnosed cardiac sarcoidosis who underwent autopsy or cardiac transplantation. Thirty‐three eligible articles provided images of 49 unique hearts. Analysis of these hearts revealed certain features of myocardial involvement in >90% of cases: left ventricular (LV) subepicardial, LV multifocal, septal, and right ventricular free wall involvement. In contrast, other patterns were seen in 0% to 6% of cases: absence of gross LV myocardial involvement, isolated LV midmyocardial involvement, isolated LV subendocardial involvement, isolated LV transmural involvement, absence of septal involvement, or isolated involvement of only one LV level.
Conclusions
In this systematic review and meta‐analysis of histologically diagnosed cardiac sarcoidosis, we identified certain features of myocardial involvement that occurred frequently and others that occurred rarely or never. These patterns could aid the interpretation of cardiovascular magnetic resonance imaging and positron emission tomography imaging and improve the diagnosis and the prognostication of patients with suspected cardiac sarcoidosis.
Keywords: autopsy, cardiac sarcoidosis, cardiac transplantation, late gadolinium enhancement, myocardial structure, phenotype, prognosis
Subject Categories: Magnetic Resonance Imaging (MRI), Nuclear Cardiology and PET, Prognosis, Diagnostic Testing, Cardiomyopathy
Short abstract
See Editorial Patel et al
Clinical Perspective
What Is New?
We performed a systematic analysis and meta‐analysis of histologically diagnosed cardiac sarcoidosis for patterns of myocardial involvement using gross pathological images from autopsy or cardiac transplantation cases.
Certain features of myocardial involvement were seen in >90% of cases: left ventricular (LV) subepicardial, LV multifocal, septal, and right ventricular free wall involvement.
Other patterns were seen in 0% to 6% of cases: absence of gross myocardial involvement, isolated LV midmyocardial involvement, isolated LV subendocardial involvement, isolated LV transmural involvement, absence of septal involvement, or isolated involvement of only one LV level.
What Are the Clinical Implications?
These patterns of myocardial involvement in cardiac sarcoidosis could aid the interpretation of cardiovascular magnetic resonance imaging and 18F‐fluorodeoxyglucose positron emission tomography imaging and improve the diagnosis and the prognostication of patients with suspected cardiac sarcoidosis.
Introduction
Sarcoidosis is a multisystem granulomatous disorder of unclear cause. The heart is involved in up to 25% of patients with sarcoidosis, and cardiac sarcoidosis is often associated with a poor prognosis.1 Cardiovascular magnetic resonance imaging (CMR) is frequently used in the evaluation of patients with suspected cardiac sarcoidosis, and myocardial involvement identified as late gadolinium enhancement (LGE) is incorporated in the various diagnostic criteria used to make the diagnosis of cardiac sarcoidosis.2, 3, 4, 5, 6 Similarly, 18F‐fluorodeoxyglucose (18F‐FDG) positron emission tomography is also often used in the evaluation and monitoring of patients with suspected cardiac sarcoidosis, with active myocardial involvement identified as 18F‐FDG uptake.2, 3, 4, 5, 6
Patel et al first described diverse patterns of LGE in patients with extracardiac biopsy‐proven sarcoidosis.7 Although 86% (18/21) of patients with LGE in the study had at least one region with LGE in a nonischemic pattern, subendocardial LGE typical for coronary artery disease was also noted as representing cardiac sarcoidosis in the absence of obstructive coronary artery disease. This study was the basis of statements in the 2014 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis that “there is no specific pattern of LGE that is pathognomonic for cardiac sarcoidosis” and “…even a pattern that is typical for prior myocardial infarction can also represent cardiac sarcoidosis.”3 The lack of a specific LGE pattern for cardiac sarcoidosis makes it challenging to make the diagnosis.8 This is an important issue as patient management is often guided by clinical diagnoses rather than histological diagnoses because an endomyocardial biopsy is deemed to have limited sensitivity to detect cardiac sarcoidosis.3 Although the presence of frequent LGE patterns in cardiac sarcoidosis has been suggested,9, 10 there has been no systematic analysis of patterns of myocardial involvement in cardiac sarcoidosis, especially in histologically diagnosed cardiac sarcoidosis. We hypothesized that certain patterns of myocardial involvement are more frequent in histologically diagnosed cardiac sarcoidosis.
To determine patterns of myocardial involvement in cardiac sarcoidosis, we performed a systematic review and meta‐analysis of published gross pathological images of the heart from patients with histologically diagnosed cardiac sarcoidosis.
Methods
All data supporting the findings are provided within the article. We studied gross pathological images of hearts from either patients who underwent a autopsy or those who had heart transplantation for cardiac sarcoidosis and had a definitive histological diagnosis of cardiac sarcoidosis. Patients who underwent a autopsy died from either sudden cardiac death attributed to cardiac sarcoidosis or other causes directly related to cardiac sarcoidosis (eg, multiorgan failure after recurrent ventricular arrhythmias attributed to cardiac sarcoidosis). We chose to specifically study these patients because they experienced the major adverse cardiac events that we aim to avoid in patients with suspected cardiac sarcoidosis.
Search Strategy
We searched the PubMed, Embase, and Cochrane databases in March 2018 to perform a systematic review of peer‐reviewed publications that included gross pathological images of hearts taken from patients who either died from cardiac sarcoidosis or underwent heart transplantation for cardiac sarcoidosis. Search terms used were as follows: “cardiac sarcoidosis and pathology,” “cardiac sarcoidosis and autopsy,” “cardiac sarcoidosis and autopsy,” and “cardiac sarcoidosis and explant.”
Study Selection
Two investigators (O.O. and C.S.) independently scanned all titles and abstracts and obtained full‐text reports of articles that indicated or suggested eligibility. The full‐text articles were then assessed for eligible gross heart pathological images by the same investigators independently. We included images from patients who either died of causes related to cardiac sarcoidosis or underwent heart transplantation for cardiac sarcoidosis and had a histological diagnosis of cardiac sarcoidosis based on the presence of noncaseating granulomas. We excluded gross pathological images when the myocardium could not be assessed in at least 6 of 17 American Heart Association left ventricular (LV) segments11 because of either anatomical sections performed or poor image quality.
Data Collection
To identify features of myocardial involvement in patients with cardiac sarcoidosis, 2 investigators (O.O., F.K.) independently recorded the following 5 domains of myocardial damage features from the gross pathological images:
-
Location of involvement within the LV wall:
Subepicardial (involvement of the outer portion, including the right ventricular [RV] aspect of the interventricular septum);
Midmyocardial (involvement of the middle portion);
Subendocardial (involvement of the inner portion);
Transmural (involvement of the entire thickness of the wall).
-
Focality within the LV:
Unifocal (1 single lesion);
Multifocal (>1 discrete lesion).
-
LV segments involved:
Anterior segments;
Septal segments;
Inferior segments;
Lateral segment.
-
LV levels involved:
Basal LV;
Mid LV;
Apical LV.
-
Involvement of the RV free wall
Yes;
No.
Discordances were resolved after consensus with a third investigator (C.S.). Within each domain, the prevalence of various features of myocardial involvement was compared. Features that were either frequently (>90%) or rarely (<10%) present were identified.
Statistical Analysis
Categorical variables were expressed as counts with percentages. χ2 Tests were used to compare discrete data between groups; in those cases in which the expected cell count was <5, the Fisher exact test was used. Statistical analyses were performed using R, version 3.3.3 (The R Foundation; https://www.r-project.org/). All statistical tests were 2 tailed, and P<0.05 was considered statistically significant.
Results
The systematic review yielded 33 articles12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 published in the peer‐reviewed literature between 1974 and March 2018 (Figure 1). The 33 articles provided gross pathological images of 49 unique hearts with cardiac sarcoidosis: 25 were from autopsy examinations, and 24 were explanted for heart transplantation (Table 1). All heart transplantations occurred for cardiac sarcoidosis, and of the 25 hearts from autopsy examinations, 18 (72%) had sudden death and 7 (28%) died of immediate causes other than sudden death but cardiac sarcoidosis directly contributed to the death. Five representative examples17, 24, 29, 39, 44 with details of features of myocardial involvement are reproduced in Figure 2. All 49 gross pathological images are reproduced with permission in Figures S1 through S49.
Figure 1.
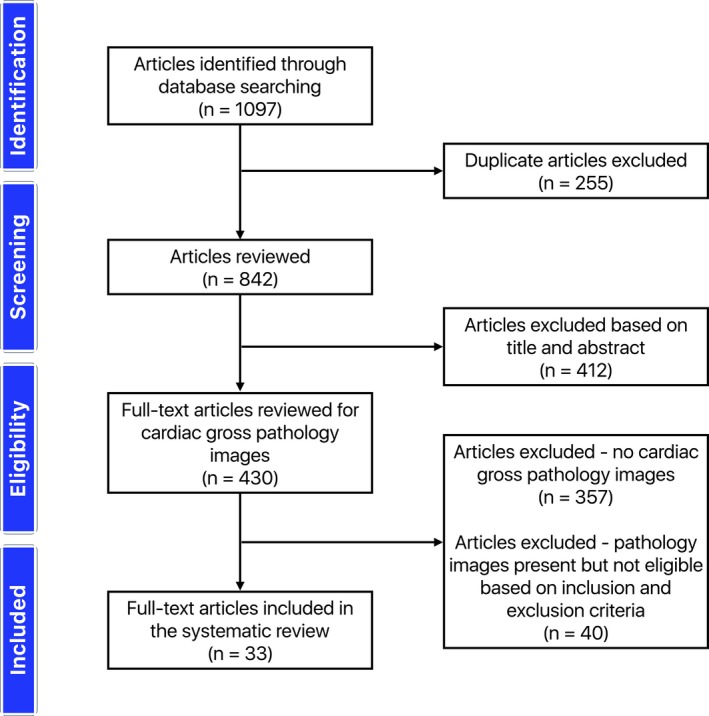
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram showing the flow of information through the different phases of the systematic review.
Table 1.
Articles and Gross Pathological Images of Cardiac Sarcoidosis Included in the Study
| Figure | Article No. | Author and Year of Publication | Figure No. Within Article | Autopsy or Explant | Cause of Death | No. of LV Segments Seen |
|---|---|---|---|---|---|---|
| S1 | 1 | Fawcett and Goldberg, 197412 | 1 | Autopsy | Sudden cardiac death | 6 |
| S2 | 2 | Fleming, 197413 | 4 | Autopsy | Sudden cardiac death | 6 |
| S3 | 2 | Fleming, 197413 | 9 | Autopsy | Sudden cardiac death | 6 |
| S4 | 3 | Roberts et al, 197714 | 5 | Autopsy | Sudden cardiac death | 6 |
| S5 | 4 | James and Pounder, 198215 | 1 | Autopsy | Sudden cardiac death | 6 |
| S6 | 5 | (Authors not listed), 199016 | 9 | Autopsy | Cardiogenic shock | 6 |
| S7 | 6 | Antecol and Roberts, 199017 | 5 | Autopsy | Sudden cardiac death | 12 |
| S8 | 7 | Shirani and Roberts, 199318 | 4 | Autopsy | Sudden cardiac death | 17 |
| S9 | 7 | Shirani and Roberts, 199318 | 5 | Autopsy | Sudden cardiac death | 6 |
| S10 | 8 | Donsky et al, 200219 | 2 | Explant | … | 17 |
| S11 | 9 | Wan Muhaizan et al, 200420 | 2 | Autopsy | Cardiogenic shock | 16 |
| S12 | 10 | Goyal and Aragam, 200621 | 1 | Explant | … | 6 |
| S13 | 11 | Halushka et al, 200622 | 1 | Explant | … | 6 |
| S14 | 12 | Hamilton et al, 200723 | 1 | Autopsy | Sudden cardiac death | 6 |
| S15 | 13 | Morikawa et al, 200824 | 2 | Autopsy | Hemorrhagic shock | 6 |
| S16 | 14 | Luk et al, 200925 | 2 | Explant | … | 6 |
| S17 | 15 | Riezzo et al, 200926 | 2 | Autopsy | Sudden cardiac death | 6 |
| S18 | 16 | Roberts et al, 200927 | 2 | Explant | … | 17 |
| S19 | 16 | Roberts et al, 200927 | 3 | Explant | … | 17 |
| S20 | 16 | Roberts et al, 200927 | 4 | Explant | … | 12 |
| S21 | 17 | Sharma et al, 200928 | 1, 2 | Autopsy | Sudden cardiac death | 17 |
| S22 | 18 | Tavora et al, 200929 | 2 | Autopsy | Sudden cardiac death | 6 |
| S23 | 18 | Tavora et al, 200929 | 2 | Autopsy | Sudden cardiac death | 6 |
| S24 | 18 | Tavora et al, 200929 | 3 | Autopsy | Sudden cardiac death | 17 |
| S25 | 18 | Tavora et al, 200929 | 7 | Autopsy | Sudden cardiac death | 6 |
| S26 | 19 | Dubrey and Falk, 201030 | 2 | Explant | … | 6 |
| S27 | 20 | Lagana et al, 201031 | 1 | Explant | … | 6 |
| S28 | 21 | Bagwan et al, 201132 | 1 | Autopsy | Sudden cardiac death | 6 |
| S29 | 22 | Strauss et al, 201133 | 2 | Explant | … | 6 |
| S30 | 23 | Armstrong, 201334 | 2 | Autopsy | Sudden cardiac death | 6 |
| S31 | 24 | Zacek et al, 201335 | 2 | Autopsy | Cardiogenic shock | 6 |
| S32 | 25 | Lynch et al, 201436 | 1 | Autopsy | Sudden cardiac death | 6 |
| S33 | 26 | Roberts et al, 2014a37 | 1 | Explant | … | 17 |
| S34 | 26 | Roberts et al, 201437 | 2 | Explant | … | 17 |
| S35 | 26 | Roberts et al, 201437 | 3 | Explant | … | 17 |
| S36 | 26 | Roberts et al, 201437 | 4 | Explant | … | 17 |
| S37 | 26 | Roberts et al, 201437 | 5 | Explant | … | 12 |
| S38 | 27 | Roberts et al, 201438 | 27 | Explant | … | 17 |
| S39 | 28 | Armstrong et al, 201539 | 1 | Explant | … | 17 |
| S40 | 28 | Armstrong et al, 201539 | 3 | Explant | … | 17 |
| S41 | 29 | Jeudy et al, 201540 | 2 | Autopsy | Sudden cardiac death | 6 |
| S42 | 29 | Jeudy et al, 201540 | 3 | Explant | … | 6 |
| S43 | 29 | Jeudy et al, 201540 | 4 | Explant | … | 6 |
| S44 | 30 | Kajimoto et al, 201541 | 2 | Autopsy | Hemorrhagic shock | 17 |
| S45 | 31 | Vasaturo et al, 201542 | 1 | Autopsy | Toxic shock syndrome | 16 |
| S46 | 32 | Di Gesaro et al, 201643 | 2 | Explant | … | 17 |
| S47 | 33 | Roberts et al, 201844 | 2 | Explant | … | 6 |
| S48 | 33 | Roberts et al, 201844 | 2 | Explant | … | 6 |
| S49 | 33 | Roberts et al, 201844 | 2 | Explant | … | 6 |
LV indicates left ventricular.
Figure 2.
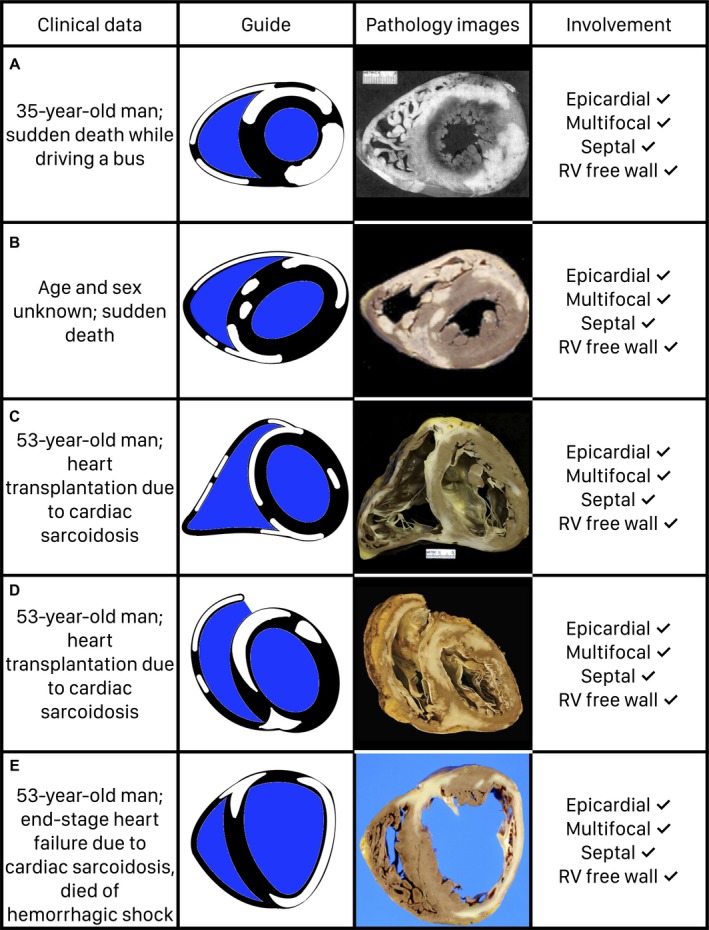
Illustrated examples of 5 gross pathological images from the study, demonstrating frequent features of myocardial involvement in cardiac sarcoidosis, are shown. A, Image is reprinted from Antecol and Roberts17 with permission. Copyright © 1990, Elsevier. B, Image is reprinted from Tavora et al29 with permission. Copyright © 2009, Elsevier. C, Image is reprinted from Armstrong et al39 with permission. Copyright © 2013, Wolters Kluwer Health, Inc. D, Image is reprinted from Roberts et al44 with permission. Copyright © 2018, American Medical Association. E, Image is reprinted from Morikawa et al24 with permission. Copyright © 2008, Elsevier. RV indicates right ventricular.
Prevalence of Features of Myocardial Involvement
Location of involvement within the LV wall
Within the LV wall, the involvement was subepicardial in 98% of cases, with significantly lower midmyocardial (65.3%), subendocardial (53.1%), or transmural (63.3%) involvement (P<0.05 for all) (Tables 2 and 3).
Table 2.
Features of Myocardial Involvement in Cardiac Sarcoidosis on Gross Pathological Images
| Figure | Subepicardial LV Involvement | Midmyocardial LV Involvement | Subendocardial LV Involvement | Transmural LV Involvement | Multifocal LV Involvement | Septal LV Involvement | Lateral LV Involvement | Anterior LV Involvement | Inferior LV Involvement | Basal LV Involvement | Mid‐LV Involvement | Apical LV Involvement | RV Free Wall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Yes | Yes | Yes | No | Yes | Yes | Yes | … | … | Yes | Yes | Yes | … |
| S2 | Yes | No | Yes | Yes | Yes | Yes | No | … | … | Yes | Yes | Yes | Yes |
| S3 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes | Yes | Yes | … |
| S4 | Yes | Yes | No | Yes | Yes | Yes | No | … | … | Yes | Yes | No | No |
| S5 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | Yes | … | Yes |
| S6 | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | … | Yes | … | Yes |
| S7 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | Yes |
| S8 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S9 | Yes | Yes | No | No | Yes | Yes | Yes | … | … | Yes | Yes | Yes | Yes |
| S10 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| S11 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| S12 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes | Yes | Yes | Yes |
| S13 | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | … | … | Yes |
| S14 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | … |
| S15 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes |
| S16 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes |
| S17 | Yes | No | Yes | No | Yes | Yes | Yes | … | … | Yes | Yes | No | … |
| S18 | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| S19 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S20 | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | Yes | … | Yes |
| S21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S22 | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | No |
| S23 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S24 | Yes | No | No | No | No | Yes | No | No | No | … | Yes | … | Yes |
| S25 | Yes | No | No | Yes | No | Yes | No | No | Yes | … | Yes | … | Yes |
| S26 | Yes | Yes | No | Yes | Yes | Yes | Yes | … | … | Yes | Yes | Yes | Yes |
| S27 | Yes | Yes | No | Yes | Yes | Yes | No | … | … | Yes | Yes | No | Yes |
| S28 | Yes | Yes | No | No | Yes | Yes | No | Yes | No | … | Yes | … | No |
| S29 | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | … | Yes | … | … |
| S30 | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | … | … | Yes |
| S31 | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | … | … | Yes |
| S32 | Yes | Yes | No | No | Yes | Yes | Yes | No | No | … | Yes | … | No |
| S33 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| S34 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S35 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S36 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S37 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | Yes |
| S38 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S39 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S40 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S41 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes |
| S42 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | … | Yes | … | Yes |
| S43 | Yes | No | No | No | No | Yes | No | Yes | No | … | Yes | … | … |
| S44 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S45 | Yes | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes |
| S46 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| S47 | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes | … | … | Yes |
| S48 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes |
| S49 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | … | … | Yes |
LV indicates left ventricular; RV, right ventricular.
Table 3.
Prevalence of Features of Myocardial Involvement in Cardiac Sarcoidosis
| Feature of Myocardial Involvement | Prevalence, No./Total (%) |
|---|---|
| LV subepicardial involvement (any) | 48/49 (98.0) |
| LV midmyocardial involvement (any) | 32/49 (65.3) |
| LV subendocardial involvement (any) | 26/49 (53.1) |
| LV transmural involvement (any) | 31/49 (63.3) |
| LV multifocal involvement | 46/49 (93.9) |
| Septal segment involvement (any) | 48/49 (98.0) |
| LV lateral segment involvement (any) | 35/49 (71.4) |
| LV anterior segment involvement (any) | 30/40 (75.0) |
| LV inferior segment involvement (any) | 33/40 (82.5) |
| Basal LV involvement (any) | 39/40 (97.5) |
| Mid‐LV involvement (any) | 38/38 (100.0) |
| Apical LV involvement (any) | 21/26 (80.8) |
| RV free wall involvement (any) | 39/43 (90.7) |
LV indicates left ventricular; RV, right ventricular.
Focality within the LV
Multifocal LV involvement was significantly more common than unifocal involvement (93.9% versus 6.1%; P<0.05).
LV segments involved
The septal segments were involved in 98% of cases, with significantly lower involvement of the anterior (75.0%), lateral (71.4%), and inferior (82.5%) segments (P<0.05 for all).
LV levels involved
The basal and mid LV were almost always involved (97.5% and 100%, respectively), and the apical LV was less often involved when compared with either basal or mid LV (80.8%; P<0.05 for both comparisons). However, the difference was not significant when comparisons were made only using the 26 patients who had pathological images of all LV levels.
Involvement of the RV free wall
The RV free wall was involved in 90.7% of cases.
Rare Features of Myocardial Involvement
On the basis of the above, we identified a list of rare features (Table 4). These features had a prevalence of <6%, and many were never present. There were no patients without gross LV myocardial involvement. LV midmyocardial or subendocardial involvement without subepicardial involvement was never present. LV transmural involvement without separate subepicardial involvement was present in only 1 patient (2.0%). Unifocal involvement was present in only 6.1% of patients. Absence of septal involvement was noted in only 1 patient (2.0%). In terms of the levels of involvement, isolated involvement of only one LV level was seen in only 1 patient (2.0%) for the basal level and 0 patients for the mid and apical levels.
Table 4.
Rare Features of Myocardial Involvement in Cardiac Sarcoidosis
| Feature of Myocardial Involvement | Prevalence, No./Total (%) |
|---|---|
| No gross LV involvement | 0/49 (0.0) |
| No LV subepicardial involvement | 1/49 (2.0) |
| LV midmyocardial involvement without subepicardial involvement | 0/49 (0.0) |
| LV subendocardial involvement without subepicardial involvement | 0/49 (0.0) |
| LV transmural involvement without separate subepicardial involvement | 1/49 (2.0) |
| LV unifocal involvement | 3/49 (6.1) |
| No septal wall involvement | 1/49 (2.0) |
| LV lateral wall involvement without septal wall involvement | 1/49 (2.0) |
| No LV basal involvement | 1/26 (3.8)a |
| No LV mid involvement | 0/26 (0.0)a |
| Apical LV involvement without basal or mid LV involvement | 0/26 (0.0)a |
LV indicates left ventricular.
A total of 26 patients had images of the basal, mid, and apical LV.
Discussion
In this systematic review and meta‐analysis of gross pathological images of hearts from patients with histologically diagnosed cardiac sarcoidosis who underwent either autopsy or heart transplantation for cardiac sarcoidosis, we identified frequent and rare features of myocardial involvement. LV subepicardial, LV multifocal, septal, and RV free wall involvement were frequent (present in >90% of patients) features. On the other hand, lack of gross LV myocardial, isolated LV midmyocardial, or isolated LV subendocardial involvement was never present. Similarly, isolated LV transmural involvement, absence of septal involvement, and isolated involvement of only one LV level were rare (present in 2% of patients) features.
One of the key strengths of our data is that they are derived only from patients with histologically diagnosed cardiac sarcoidosis. The contemporary understanding of myocardial involvement in cardiac sarcoidosis, in which no specific patterns are believed to be pathognomonic for cardiac sarcoidosis, is largely based on LGE CMR data,7 which include patients with clinical but not histologically diagnosed cardiac sarcoidosis. In these studies, a clinical diagnosis of cardiac sarcoidosis is reached after excluding other explanations for the LGE,3 which may not always be accurate. For instance, coronary artery disease as the cause for subendocardial LGE in patients with suspected cardiac sarcoidosis is typically excluded by the absence of obstructive coronary artery disease on coronary angiography.7 However, this does not exclude the possibility of myocardial infarction with nonobstructive coronary arteries.45, 46
Our data demonstrate that there are characteristic features of myocardial involvement in cardiac sarcoidosis. These features could be used to identify patients with cardiac sarcoidosis using LGE CMR and 18F‐FDG positron emission tomography, particularly those in whom cardiac sarcoidosis was not suspected before the imaging study. More important, these data imply that patients with LGE or 18F‐FDG uptake in patterns that were never or rarely present in this systematic review could have an alternate explanation for the imaging findings. For instance, isolated subendocardial LGE may represent a myocardial infarction, and in the absence of coronary artery disease, it may still represent myocardial infarction with nonobstructive coronary arteries, rather than cardiac sarcoidosis. Similarly, 18F‐FDG uptake isolated to the lateral wall may represent inadequate suppression of physiological uptake rather than true cardiac sarcoidosis.47
Limitations
Our systematic review and meta‐analysis is based on the published pathological literature, which introduces bias. Only a third of cases had 16 or 17 segments included in the gross pathological images, which raises the possibility that some of the features of myocardial involvement could have been missed. Cases included in the publications represent the most impressive cases and may not be representative of the entire spectrum of pathologically identified myocardial involvement in cardiac sarcoidosis. Similarly, our systematic review focuses on end‐stage cardiac sarcoidosis (ie, those who either died of cardiac sarcoidosis or underwent heart transplantation because of it). Thus, it could be argued that our data do not include features of early myocardial involvement in cardiac sarcoidosis. However, our cases represent the adverse outcomes that we aim to avoid in patients with suspected cardiac sarcoidosis (ie, cardiac death and heart transplantation). Therefore, our data may carry prognostic implications. Studies are ongoing using these patterns of myocardial involvement on LGE CMR or 18F‐FDG to risk stratify patients with suspected cardiac sarcoidosis.
Conclusions
Myocardial involvement in end‐stage cardiac sarcoidosis involves frequent (LV subepicardial, LV multifocal, septal, and RV free wall involvement) and rare (lack of gross LV myocardial involvement, isolated LV midmyocardial involvement, isolated LV subendocardial involvement, isolated LV transmural involvement, absence of septal involvement, or isolated involvement of only one LV level) features. These patterns could be used to improve diagnosis and prognostication of suspected cardiac sarcoidosis with noninvasive imaging modalities, such as LGE CMR and 18F‐FDG positron emission tomography.
Disclosures
None.
Sources of Funding
Chetan Shenoy was supported by NIH grant K23HL132011 and a University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (NIH grant KL2TR000113‐05).
Supporting information
Figure S1. See Table 2 for interpretation of cardiac involvement. Reprinted from Fawcett et al1 with permission.
Figure S2. See Table 2 for interpretation of cardiac involvement. Reprinted from Fleming et al2 with permission. Copyright ©1974, BMJ Publishing Group Ltd.
Figure S3. See Table 2 for interpretation of cardiac involvement. Reprinted from Fleming et al2 with permission. Copyright ©1974, BMJ Publishing Group Ltd.
Figure S4. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al3 with permission. Copyright ©1977, Elsevier.
Figure S5. See Table 2 for interpretation of cardiac involvement. Reprinted with permission from James et al4 with permission. Copyright ©1982, Elsevier.
Figure S6. See Table 2 for interpretation of cardiac involvement. Reprinted from [authors not listed]5 with permission. Copyright ©1990, Elsevier.
Figure S7. See Table 2 for interpretation of cardiac involvement. Reprinted from Antecol et al6 with permission. Copyright ©1990, Elsevier.
Figure S8. See Table 2 for interpretation of cardiac involvement. Reprinted from Shirani et al7 with permission. Copyright ©1993, Elsevier.
Figure S9. See Table 2 for interpretation of cardiac involvement. Reprinted from Shirani et al7 with permission. Copyright ©1993, Elsevier.
Figure S10. See Table 2 for interpretation of cardiac involvement. Reprinted from Donsky et al8 with permission. Copyright ©2008, Elsevier.
Figure S11. See Table 2 for interpretation of cardiac involvement. Reprinted from Wan Muhaizan et al9 with permission. Copyright ©2004, Malaysian Society of Pathologists.
Figure S12. See Table 2 for interpretation of cardiac involvement. Reprinted from Goyal et al10 with permission. Copyright ©2006, Wolters Kluwer Health, Inc.
Figure S13. See Table 2 for interpretation of cardiac involvement. Reprinted from Halushka et al11 with permission. Copyright ©2006, Elsevier.
Figure S14. See Table 2 for interpretation of cardiac involvement. Reprinted from Hamilton et al12 with permission. Copyright ©2007, John Wiley and Sons.
Figure S15. See Table 2 for interpretation of cardiac involvement. Reprinted from Morikawa et al13 with permission. Copyright ©2008, Elsevier.
Figure S16. See Table 2 for interpretation of cardiac involvement. Reprinted from Luk et al14 with permission. Copyright ©2009, Elsevier.
Figure S17. See Table 2 for interpretation of cardiac involvement. Reprinted from Riezzo et al15 with permission. Copyright ©2009, Elsevier.
Figure S18. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S19. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S20. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S21. See Table 2 for interpretation of cardiac involvement. Reprinted from Sharma et al17 with permission. Copyright ©2009, Taylor & Francis.
Figure S22. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S23. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S24. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S25. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S26. See Table 2 for interpretation of cardiac involvement. Reprinted from Dubrey et al19 with permission. Copyright ©2010, Elsevier.
Figure S27. See Table 2 for interpretation of cardiac involvement. Reprinted from Lagana et al20 with permission. Copyright ©2010, College of American Pathologists.
Figure S28. See Table 2 for interpretation of cardiac involvement. Reprinted from Bagwan et al21 with permission. Copyright ©2011, Springer Nature.
Figure S29. See Table 2 for interpretation of cardiac involvement. Reprinted from Strauss et al22 with permission. Copyright ©2011, John Wiley and Sons.
Figure S30. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong23 with permission. Copyright ©2013, Wolters Kluwer Health, Inc.
Figure S31. See Table 2 for interpretation of cardiac involvement. Reprinted from Zacek et al24 with permission. Copyright ©2013, John Wiley and Sons.
Figure S32. See Table 2 for interpretation of cardiac involvement. Reprinted from Lynch et al25 with permission. Copyright ©2014, Georg Thieme Verlag KG.
Figure S33. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S34. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S35. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S36. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S37. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S38. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al27 with permission. Copyright ©2014, Wolters Kluwer Health, Inc.
Figure S39. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong et al28 with permission. Copyright© 2015, Elsevier.
Figure S40. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong et al28 with permission. Copyright© 2015, Elsevier.
Figure S41. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S42. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S43. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S44. See Table 2 for interpretation of cardiac involvement. Reprinted from Kajimoto et al30 with permission. Copyright ©2015, John Wiley and Sons.
Figure S45. See Table 2 for interpretation of cardiac involvement. Reprinted from Vasaturo et al31 with permission. Copyright ©2015, The Korean Society of Radiology.
Figure S46. See Table 2 for interpretation of cardiac involvement. Reprinted from Di Gesaro et al32 with permission. Copyright ©2016, Elsevier.
Figure S47. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.
Figure S48. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.
Figure S49. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.
(J Am Heart Assoc. 2019;8:e011253 DOI: 10.1161/JAHA.118.011253.)
References
- 1. Sauer WH, Stern BJ, Baughman RP, Culver DA, Royal W. High‐risk sarcoidosis: current concepts and research imperatives. Ann Am Thorac Soc. 2017;14:S437–S444. [DOI] [PubMed] [Google Scholar]
- 2. Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 3. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 4. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, Sweiss N, Crouser E, Morgenthau AS, Lower EE, Azuma A, Ishihara M, Morimoto S, Tetsuo Yamaguchi T, Shijubo N, Grutters JC, Rosenbach M, Li HP, Rottoli P, Inoue Y, Prasse A, Baughman RP; Organ Assessment Instrument Investigators TW . The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 5. Moller DR, Koth LL, Maier LA, Morris A, Drake W, Rossman M, Leader JK, Collman RG, Hamzeh N, Sweiss NJ, Zhang Y, O'Neal S, Senior RM, Becich M, Hochheiser HS, Kaminski N, Wisniewski SR, Gibson KF; GRADS Sarcoidosis Study Group . Rationale and design of the Genomic Research in Alpha‐1 Antitrypsin Deficiency and Sarcoidosis (GRADS) Study: sarcoidosis protocol. Ann Am Thorac Soc. 2015;12:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol. 2017;3:42–45. [Google Scholar]
- 7. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blankstein R, Kramer CM, Chandrashekhar Y. The challenges of diagnosing cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:1534–1536. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe E, Kimura F, Nakajima T, Hiroe M, Kasai Y, Nagata M, Kawana M, Hagiwara N. Late gadolinium enhancement in cardiac sarcoidosis: characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging. 2013;28:60–66. [DOI] [PubMed] [Google Scholar]
- 10. Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long‐term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging. 2015;16:634–641. [DOI] [PubMed] [Google Scholar]
- 11. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 12. Fawcett FJ, Goldberg MJ. Heart block resulting from myocardial sarcoidosis. Br Heart J. 1974;36:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleming HA. Sarcoid heart disease. Br Heart J. 1974;36:54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 15. James RA, Pounder DJ. Cardiac sarcoidosis with spontaneous rupture of the right ventricle. Forensic Sci Int. 1982;20:167–171. [DOI] [PubMed] [Google Scholar]
- 16. Refractory ventricular arrhythmias and death in a 43‐year‐old man. Am J Med. 1990;89:496–506. [DOI] [PubMed] [Google Scholar]
- 17. Antecol DH, Roberts WC. Sudden death behind the wheel from natural disease in drivers of four‐wheeled motorized vehicles. Am J Cardiol. 1990;66:1329–1335. [DOI] [PubMed] [Google Scholar]
- 18. Shirani J, Roberts WC. Subepicardial myocardial lesions. Am Heart J. 1993;125:1346–1352. [DOI] [PubMed] [Google Scholar]
- 19. Donsky AS, Escobar J, Capehart J, Roberts WC. Heart transplantation for undiagnosed cardiac sarcoidosis. Am J Cardiol. 2002;89:1447–1450. [DOI] [PubMed] [Google Scholar]
- 20. Wan Muhaizan WM, Swaminathan M, Daud MS. Cardiac sarcoidosis: two cases with autopsy findings. Malays J Pathol. 2004;26:59–63. [PubMed] [Google Scholar]
- 21. Goyal SB, Aragam JR. Cardiac sarcoidosis with primary involvement of the tricuspid valve. Cardiol Rev. 2006;14:e12–e13. [DOI] [PubMed] [Google Scholar]
- 22. Halushka MK, Yuh DD, Russell SD. Right ventricle‐dominant cardiac sarcoidosis with sparing of the left ventricle. J Heart Lung Transplant. 2006;25:479–482. [DOI] [PubMed] [Google Scholar]
- 23. Hamilton RA, Sullivan L, Wolf BC. Sudden cardiac death due to giant cell inflammatory processes. J Forensic Sci. 2007;52:943–948. [DOI] [PubMed] [Google Scholar]
- 24. Morikawa M, Kato K, Kako N, Hiramitsu S, Oguri M, Yajima K, Hibino T, Kato Y, Mizoguchi Y, Kuroda M, Yokoi K, Ichiro Morimoto S. A failed case to diagnose cardiac sarcoidosis presenting advanced atrioventricular block. Int J Cardiol. 2008;129:e46–e49. [DOI] [PubMed] [Google Scholar]
- 25. Luk A, Metawee M, Ahn E, Gustafsson F, Ross H, Butany J. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol. 2009;25:e48–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riezzo I, Ventura F, D'Errico S, Neri M, Turillazzi E, Fineschi V. Arrhythmogenesis and diagnosis of cardiac sarcoidosis: an immunohistochemical study in a sudden cardiac death. Forensic Sci Int. 2009;183:e1–e5. [DOI] [PubMed] [Google Scholar]
- 27. Roberts WC, Vowels TJ, Ko JM, Capehart JE, Hall SA. Cardiac transplantation for cardiac sarcoidosis with initial diagnosis by examination of the left ventricular apical “core” excised for insertion of a left ventricular assist device for severe chronic heart failure. Am J Cardiol. 2009;103:110–114. [DOI] [PubMed] [Google Scholar]
- 28. Sharma PS, Lubahn JG, Donsky AS, Yoon AD, Carry MM, Grayburn PA, Wood PB, Ko JM, Burton EC, Roberts WC. Diagnosing cardiac sarcoidosis clinically without tissue confirmation. Proc (Bayl Univ Med Cent). 2009;22:236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–577. [DOI] [PubMed] [Google Scholar]
- 30. Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–346. [DOI] [PubMed] [Google Scholar]
- 31. Lagana SM, Parwani AV, Nichols LC. Cardiac sarcoidosis: a pathology‐focused review. Arch Pathol Lab Med. 2010;134:1039–1046. [DOI] [PubMed] [Google Scholar]
- 32. Bagwan IN, Hooper LV, Sheppard MN. Cardiac sarcoidosis and sudden death: the heart may look normal or mimic other cardiomyopathies. Virchows Arch. 2011;458:671–678. [DOI] [PubMed] [Google Scholar]
- 33. Strauss DG, Selvester RH, Dibernardo LR. Myocardial scar in sarcoidosis by 12‐lead ECG and pathology. Ann Noninvasive Electrocardiol. 2011;16:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armstrong EJ. Multivisceral sarcoidosis: an unexpected finding in a water‐related death. Am J Forensic Med Pathol. 2013;34:11–15. [DOI] [PubMed] [Google Scholar]
- 35. Zacek P, Omran N, Chek JL, Krbal L, Vojacek J, Harrer J. Cardiac sarcoidosis. J Card Surg. 2013;28:525–528. [DOI] [PubMed] [Google Scholar]
- 36. Lynch JP III, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014;35:372–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts WC, Chung MS, Ko JM, Capehart JE, Hall SA. Morphologic features of cardiac sarcoidosis in native hearts of patients having cardiac transplantation. Am J Cardiol. 2014;113:706–712. [DOI] [PubMed] [Google Scholar]
- 38. Roberts WC, Roberts CC, Ko JM, Filardo G, Capehart JE, Hall SA. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence or incongruence between the clinical and morphologic diagnoses. Medicine (Baltimore). 2014;93:211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armstrong D, Gonzalez‐Stawinski GV, Ko JM, Hall SA, Roberts WC. The two extremes of cardiac sarcoidosis and the effect of prednisone therapy. Am J Cardiol. 2015;115:150–153. [DOI] [PubMed] [Google Scholar]
- 40. Jeudy J, Burke AP, White CS, Kramer GB, Frazier AA. Cardiac sarcoidosis: the challenge of radiologic‐pathologic correlation: from the radiologic pathology archives. Radiographics. 2015;35:657–679. [DOI] [PubMed] [Google Scholar]
- 41. Kajimoto N, Hao H, Kawakami R, Takagi Y, Fujino A, Sugahara M, Masuyama T, Hirota S. Cardiac sarcoidosis predominantly involved in right ventricle: an necropsy case. Pathol Int. 2015;65:619–621. [DOI] [PubMed] [Google Scholar]
- 42. Vasaturo S, Ploeg DE, Buitrago G, Zeppenfeld K, Veselic‐Charvat M, Kroft LJ. Right ventricular cardiomyopathy meeting the arrhythmogenic right ventricular dysplasia revised criteria? Don't forget sarcoidosis! Korean J Radiol. 2015;16:668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Gesaro G, Tortorici E, Bellavia D, Licata P, Chiarello G, Liotta R, Scardulla C, Clemenza F. Cardiac sarcoidosis: matching speckle tracking echocardiography to macroscopic ventricular pathology (a case report). Int J Cardiol. 2016;203:753–756. [DOI] [PubMed] [Google Scholar]
- 44. Roberts WC, Becker TM, Hall SA. Usefulness of total 12‐lead QRS voltage as a clue to diagnosis of patients with cardiac sarcoidosis severe enough to warrant orthotopic heart transplant. JAMA Cardiol. 2018;3:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 46. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;138:e618–e651. [Google Scholar]
- 47. Ishida Y, Yoshinaga K, Miyagawa M, Moroi M, Kondoh C, Kiso K, Kumita S. Recommendations for (18)F‐fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014;28:393–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. See Table 2 for interpretation of cardiac involvement. Reprinted from Fawcett et al1 with permission.
Figure S2. See Table 2 for interpretation of cardiac involvement. Reprinted from Fleming et al2 with permission. Copyright ©1974, BMJ Publishing Group Ltd.
Figure S3. See Table 2 for interpretation of cardiac involvement. Reprinted from Fleming et al2 with permission. Copyright ©1974, BMJ Publishing Group Ltd.
Figure S4. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al3 with permission. Copyright ©1977, Elsevier.
Figure S5. See Table 2 for interpretation of cardiac involvement. Reprinted with permission from James et al4 with permission. Copyright ©1982, Elsevier.
Figure S6. See Table 2 for interpretation of cardiac involvement. Reprinted from [authors not listed]5 with permission. Copyright ©1990, Elsevier.
Figure S7. See Table 2 for interpretation of cardiac involvement. Reprinted from Antecol et al6 with permission. Copyright ©1990, Elsevier.
Figure S8. See Table 2 for interpretation of cardiac involvement. Reprinted from Shirani et al7 with permission. Copyright ©1993, Elsevier.
Figure S9. See Table 2 for interpretation of cardiac involvement. Reprinted from Shirani et al7 with permission. Copyright ©1993, Elsevier.
Figure S10. See Table 2 for interpretation of cardiac involvement. Reprinted from Donsky et al8 with permission. Copyright ©2008, Elsevier.
Figure S11. See Table 2 for interpretation of cardiac involvement. Reprinted from Wan Muhaizan et al9 with permission. Copyright ©2004, Malaysian Society of Pathologists.
Figure S12. See Table 2 for interpretation of cardiac involvement. Reprinted from Goyal et al10 with permission. Copyright ©2006, Wolters Kluwer Health, Inc.
Figure S13. See Table 2 for interpretation of cardiac involvement. Reprinted from Halushka et al11 with permission. Copyright ©2006, Elsevier.
Figure S14. See Table 2 for interpretation of cardiac involvement. Reprinted from Hamilton et al12 with permission. Copyright ©2007, John Wiley and Sons.
Figure S15. See Table 2 for interpretation of cardiac involvement. Reprinted from Morikawa et al13 with permission. Copyright ©2008, Elsevier.
Figure S16. See Table 2 for interpretation of cardiac involvement. Reprinted from Luk et al14 with permission. Copyright ©2009, Elsevier.
Figure S17. See Table 2 for interpretation of cardiac involvement. Reprinted from Riezzo et al15 with permission. Copyright ©2009, Elsevier.
Figure S18. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S19. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S20. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al16 with permission. Copyright ©2009, Elsevier.
Figure S21. See Table 2 for interpretation of cardiac involvement. Reprinted from Sharma et al17 with permission. Copyright ©2009, Taylor & Francis.
Figure S22. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S23. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S24. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S25. See Table 2 for interpretation of cardiac involvement. Reprinted from Tavora et al18 with permission. Copyright ©2009, Elsevier.
Figure S26. See Table 2 for interpretation of cardiac involvement. Reprinted from Dubrey et al19 with permission. Copyright ©2010, Elsevier.
Figure S27. See Table 2 for interpretation of cardiac involvement. Reprinted from Lagana et al20 with permission. Copyright ©2010, College of American Pathologists.
Figure S28. See Table 2 for interpretation of cardiac involvement. Reprinted from Bagwan et al21 with permission. Copyright ©2011, Springer Nature.
Figure S29. See Table 2 for interpretation of cardiac involvement. Reprinted from Strauss et al22 with permission. Copyright ©2011, John Wiley and Sons.
Figure S30. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong23 with permission. Copyright ©2013, Wolters Kluwer Health, Inc.
Figure S31. See Table 2 for interpretation of cardiac involvement. Reprinted from Zacek et al24 with permission. Copyright ©2013, John Wiley and Sons.
Figure S32. See Table 2 for interpretation of cardiac involvement. Reprinted from Lynch et al25 with permission. Copyright ©2014, Georg Thieme Verlag KG.
Figure S33. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S34. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S35. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S36. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S37. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al26 with permission. Copyright ©2014, Elsevier.
Figure S38. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al27 with permission. Copyright ©2014, Wolters Kluwer Health, Inc.
Figure S39. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong et al28 with permission. Copyright© 2015, Elsevier.
Figure S40. See Table 2 for interpretation of cardiac involvement. Reprinted from Armstrong et al28 with permission. Copyright© 2015, Elsevier.
Figure S41. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S42. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S43. See Table 2 for interpretation of cardiac involvement. Reprinted from Jeudy et al29 with permission. Copyright ©2015, Radiological Society of North America.
Figure S44. See Table 2 for interpretation of cardiac involvement. Reprinted from Kajimoto et al30 with permission. Copyright ©2015, John Wiley and Sons.
Figure S45. See Table 2 for interpretation of cardiac involvement. Reprinted from Vasaturo et al31 with permission. Copyright ©2015, The Korean Society of Radiology.
Figure S46. See Table 2 for interpretation of cardiac involvement. Reprinted from Di Gesaro et al32 with permission. Copyright ©2016, Elsevier.
Figure S47. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.
Figure S48. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.
Figure S49. See Table 2 for interpretation of cardiac involvement. Reprinted from Roberts et al33 with permission. Copyright ©2018, American Medical Association.


