Figure 1.
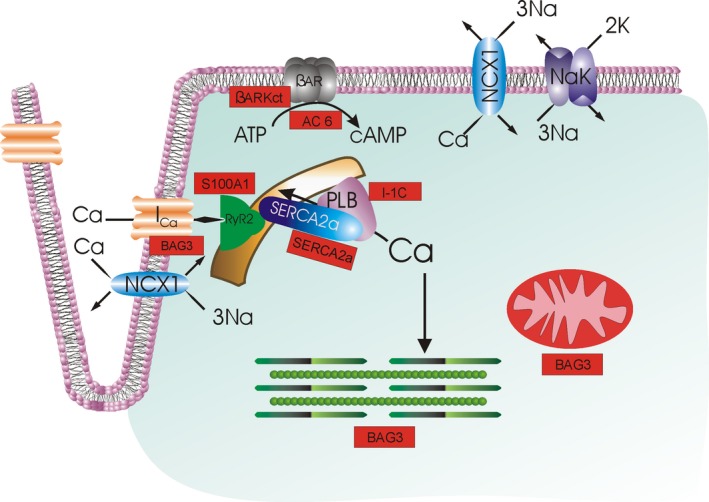
Current heart failure gene therapy approaches targeted to cardiac excitation‐contraction coupling. With depolarization, extracellular Ca2+ enters by L‐type Ca2+ channels (IC a), triggering Ca2+ release from the ryanodine receptor (RyR2) in the sarcoplasmic reticulum (SR). Ca2+ in the sarcoplasm binds to troponin to initiate contraction. During diastole, Ca2+ is resequestered in the SR by SR Ca2+‐ATPase (SERCA2a), whose activity is regulated by phospholamban (PLB). The amount of Ca2+ that has entered during systole is largely extruded by Na+/Ca2+ exchanger (NCX1; utilizing the electrochemical gradient established by Na+‐K+‐ATPase NaK) and, to a much smaller extent, the sarcolemmal Ca2+‐ATPase (not shown). When β‐adrenergic receptor (βAR) is stimulated, cAMP is generated, which activates protein kinase A (PKA), which, in turn, increases IC a and RyR2 activities and Ca2+ sensitivity of myofilaments, thereby enhancing contractility. PKA also phosphorylates PLB, thereby relieving its inhibition on SERCA2a, resulting in enhanced SR Ca2+ uptake, which improves both contraction (larger SR Ca2+ content leading to larger intracellular Ca2+ transients) and relaxation (faster SR Ca2+ sequestration during diastole). Current gene therapy products (shown in rectangular red boxes) target βAR (AC6, βARKct), SR Ca2+ uptake (SERCA2a), PLB (I‐1c), and Ca2+ cycling by RyR2 and SERCA2a (S100A1). BAG3 has multiple downstream effectors, including IC a, myofilaments, and mitochondria; not shown are autophagy, nuclear envelope integrity, and cell‐to‐cell communication (connexin43), also positively regulated by BAG3. Urocortins effect mainly vasodilation and are not shown here. SERCA2a indicates sarcoplasmic/endoplasmic reticulum calcium ATPase 2a.
