Abstract
Background
The association between time to reperfusion and clinical outcome is well known in anterior circulation strokes, whereas the impact of main time metrics remains unknown in posterior circulation strokes. We investigated the clinical effect of different time intervals from symptom onset to reperfusion on the 90‐day clinical outcome in acute ischemic stroke patients with basilar artery occlusion, and especially in the subset population presenting a low stroke volume on baseline diffusion‐weighted imaging.
Methods and Results
We studied patients included in the prospective, multicenter, observational ETIS (Endovascular Treatment in Ischemic Stroke) registry who had had basal artery occlusion and had achieved successful reperfusion (modified Thrombolysis In Cerebral Infarction 2b‐3). Three time intervals (onset to reperfusion, onset to imaging, and imaging to reperfusion) were considered in all patients and separately in patients with pc‐ASPECTS (posterior‐circulation Alberta Stroke Program Early Computed Tomography Score) <8 and ≥8 on baseline diffusion‐weighted imaging. The primary end point was good outcome defined as 90‐day modified Rankin Scale scores of 0 to 2. Among the 95 included patients, 38 (40%) achieved a good outcome. In all patients, no significant association was found between the different time intervals and outcome. In patients evaluated with diffusion‐weighted imaging (n=61) at baseline, a significant negative association was found between imaging‐to‐reperfusion time for patients with pc‐ASPECTS <8 (adjusted odds ratio=0.4 per 30‐minute increase; 95% CI 0.18‐0.85; P=0.02) compared with those with pc‐ASPECTS ≥8.
Conclusions
In patients with basilar artery occlusion and pc‐ASPECTS <8 at baseline diffusion‐weighted imaging, clinical outcome is highly dependent on the time from imaging to reperfusion, which suggests that rapid endovascular reperfusion should be performed after imaging in these patients.
Keywords: outcome, posterior circulation, reperfusion, thrombectomy
Subject Categories: Quality and Outcomes, Health Services, Revascularization, Ischemic Stroke
Clinical Perspective
What Is New?
In acute ischemic stroke patients presenting with basilar artery occlusion, time from imaging to reperfusion is a crucial factor impacting the 90‐day clinical outcome because a good clinical outcome may be achieved after rapid successful endovascular reperfusion in the setting of extensive baseline ischemia (defined as a baseline diffusion‐weighted imaging posterior‐circulation ASPECTS [Alberta Stroke Program Early Computed Tomography Score] <8).
Thus, our data confirm the evidence for posterior‐circulation strokes as reported in anterior‐circulation large‐vessel strokes.
What Are the Clinical Implications?
An aggressive target time metric in endovascular therapy for patients with a posterior‐circulation stroke should be obtained.
Patients with low pc‐ASPECTS on baseline diffusion‐weighted imaging should not be exclude from endovascular therapy.
Introduction
The clinical benefit of modern endovascular thrombectomy in acute ischemic stroke patients with proximal intracranial occlusions of the anterior circulation has recently been demonstrated through several interventional clinical trials.1 Two randomized controlled trials are testing the benefit of endovascular thrombectomy in patients with basilar thrombosis but have not yet provided final results: the BEST (Acute Basilar Artery Occlusion: Endovascular Interventions versus Standard Medical Treatment) trial2 and the BASICS (Basilar Artery International Cooperation Study) trial.3 No clinical benefit was observed in the patients treated by endovascular therapy in the BASICS registry.4 However, patients were treated with old‐generation mechanical devices, which were less effective, and with time delay between imaging and reperfusion. Some authors showed improved outcomes in patients treated with more recent mechanical endovascular devices.5 However the prognosis for basilar artery occlusion (BAO) patients remains poor, leading to death or dramatic disability,3 even after reperfusion achieved by modern endovascular techniques.6, 7 Therefore, a knowledge of those factors influencing outcome appears crucial in order to improve the clinical benefit of reperfusion.8, 9 Although the impact of workflow intervals to treat large artery occlusions of the anterior circulation has been demonstrated,10 their impact on stroke patients with BAO is not clear.
The aim of this study was to investigate the effect of different time intervals between symptom onset and reperfusion on clinical outcome at 90 days in BAO patients achieving successful reperfusion after endovascular thrombectomy using data from the large prospective multicenter ETIS (Endovascular Treatment in Ischemic Stroke) cohort, especially in the subset of the population demonstrating a large stroke volume at admission.
Methods
According to the Transparency and Openness Promotion Guidelines, the data, analytic methods, and study materials will be made available to other researchers by the corresponding author on reasonable request for purposes of reproducing the results or replicating the procedure.
Study Population
Patients included in the study were from the multicenter, observational real‐world ETIS registry, which prospectively collected data on all adult strokes treated with thrombectomy in 3 comprehensive stroke centers (Rothschild Foundation, Foch Hospital, and Pierre Wertheimer Hospital) between March 2010 and October 2017. For this study we included patients presenting with an acute BAO proven by angiography and having achieved successful reperfusion, defined as mTICI (modified Thrombolysis In Cerebral Infarction) 2b‐3 at the end of endovascular thrombectomy. Detailed materials and methods have previously been described.11 The ethics committee of each participating center approved this study, and subjects gave informed consent.
Outcomes
Baseline characteristics, pretreatment imaging findings (pc‐ASPECTS [posterior‐circulation Alberta Stroke Program Early Computed Tomography Score] on diffusion‐weighted imaging [DWI]), prior thrombolysis, all workflow times (onset to imaging, onset to reperfusion, and imaging to reperfusion), stroke etiology (according to the trial of ORG 10 17212 in acute stroke treatment classification), intervention (mTICI), and clinical‐imaging outcomes (National Institutes of Health Stroke score, intracranial hemorrhage at 24 hours, and modified Rankin Scale score at 90 days) were collected. Imaging analysis was performed by 1 operator in each comprehensive center blinded to the clinical outcome.
The primary end point was a good outcome defined by a modified Rankin Scale 0 to 2 at 90 days. A neuroradiologist calculated the DWI pc‐ASPECTS on the pretreatment magnetic resonance imaging (MRI) while blinded to the results of the endovascular procedure.13, 14 Each ASPECTS region was scored 0 if abnormal and 1 if normal. To be considered as abnormal, a DWI hyperintense signal had to be confluent. A small hyperintense speck was not enough. Both arterial occlusion site and status were monitored with conventional angiography during the endovascular therapy. Reperfusion results were reported using the mTICI score and were defined as ranging from no reperfusion (mTICI 0) to complete reperfusion (mTICI 3), including partial reperfusion (mTICI 2). For each patient the final mTICI score was retrospectively assessed by a neurointerventionalist blinded to the clinical outcome. Successful reperfusion was defined as a final mTICI score 2b‐3. All patients had a computed tomography or an MRI 24 hours after thrombectomy onset to assess hemorrhagic complications. Functional independence and mortality were assessed at 90 days during face‐to‐face interviews or via telephone calls by trained research nurses unaware of the study group assignments.15
A cutoff of pc‐ASPECTS <8 on baseline DWI was chosen as the absence of extensive ischemia according to the reported results of several studies evaluating the impact of baseline ischemia on BAO outcomes.14, 16
Statistical Analyses
Quantitative variables were expressed as means (SDs) or medians (interquartile range [IQR]) for nonnormal distributions. Normality of distributions was assessed graphically and by application of the Shapiro‐Wilk test. Categorical variables were expressed as frequencies and percentages. We assessed the association of time intervals from symptom onset to reperfusion with a good outcome (90‐day modified Rankin Scale ≤2) using logistic regression models. The shapes of relationships were examined, first by time intervals categorized into tertiles (odds ratios [ORs] for the upper tertiles of time intervals relative to the lowest were calculated, and a Cochran‐Armitage trend test was performed) and then via a graphical approach using nonparametric smoothing techniques. Because we found no evidence of a non–log‐linear relationship between time intervals and favorable outcome, the effects of time intervals were expressed as ORs and 95% CIs associated with a 30‐minute increase (derived from logistic regression model). We used multivariable logistic regression models to adjust the association between time intervals and favorable outcome on the following prespecified confounding factors: age, baseline National Institutes of Health Stroke Score, and reperfusion grade (mTICI 2b versus mTICI 3). Finally, among MRI‐based treated patients, we assessed heterogeneity in association with time intervals and favorable outcome across pc‐ASPECTS subgroups (pc‐ASPECTS <8 versus pc‐ASPECTS ≥8) on admission DWI by including an interaction term in logistic regression models. Statistical testing was performed at the 2‐tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics, revascularization status, and clinical outcomes of the included patients are reported in Table 1. During the study period, a total of 126 patients with acute BAO were treated by endovascular thrombectomy (stent retriever and/or contact aspiration). Of these, 31 were excluded from analysis due to no or poor reperfusion (n=25), missing data about time to treatment (n=3), or outcome (n=3). Among the 95 included patients, 61 underwent MRI with DWI sequences as pretreatment imaging, and 33 of them presented a DWI pc‐ASPECTS <8. Median time from onset to reperfusion was 369 minutes (IQR 268‐405); 52 (54.7%, 95% CI 35% to 55.8%) presented a reperfusion with mTICI 3, and 38 (40.0%, 95% CI 30.0% to 50.6%) a good clinical outcome at 90 days. Intracranial hemorrhage within 24 hours following treatment occurred in 13.6% of patients (95% CI 6.9% to 23%), and a mortality of 31.6% (95% CI 22.4% to 41.9%) was observed. Four and 2 patients presented DWI pc‐ASPECTS 4 and 5, respectively. A good outcome was achieved in 1 (25%) patient in the DWI pc‐ASPECTS=4 group and in 1 (50%) in the DWI pc‐ASPECTS=5 group.
Table 1.
Population Characteristics
| Variables | n | Values |
|---|---|---|
| Demographic characteristics | ||
| Age, y | 95 | 65.1 (15.7) |
| Men | 95 | 54 (56.8) |
| Medical history | ||
| Hypertension | 94 | 46 (48.9) |
| Hypercholesterolemia | 93 | 26 (28.0) |
| Diabetes mellitus | 94 | 14 (14.9) |
| Current smoker | 87 | 23 (26.4) |
| Antithrombotic medications | 95 | 33 (34.7) |
| Antiplatelet drugs | 20 (21.1) | |
| Anticoagulant drugs | 13 (13.7) | |
| Admission NIHSS scorea | 93 | 16 (10‐33) |
| MRI | 95 | 66 (69.5) |
| DWI pc‐ASPECTS <8 | 61 | 33 (54.1) |
| Etiology | 84 | |
| Large‐artery atherosclerosis | 24 (28.6) | |
| Cardioembolic | 32 (38.1) | |
| Others | 28 (33.3) | |
| Treatment details | ||
| Prior use of thrombolysis | 95 | 47 (49.5) |
| Onset to thrombolysis time, minb | 46 | 135 (162‐230) |
| Onset to imaging, min | 88 | 137 (104‐221) |
| Onset to groin puncture time, min | 95 | 302 (220‐405) |
| General anesthesia | 95 | 78 (82.1) |
| First‐line thrombectomy strategy | 95 | |
| Contact aspiration | 50 (52.6) | |
| Stent retriever | 45 (47.4) | |
| Number of passes | 95 | |
| 1 | 50 (52.6) | |
| 2 | 22 (23.2) | |
| 3 | 12 (12.6) | |
| >3c | 11 (11.6) | |
| Outcome | ||
| mTICI 3 | 95 | 52 (54.7) |
| Onset to reperfusion time, min | 95 | 369 (268‐405) |
| Imaging to reperfusion time, min | 88 | 204 (145‐272) |
| Imaging to groin puncture, min | 88 | 141 (90‐188) |
| Groin puncture to reperfusion time, min | 95 | 52 (37‐78) |
| Per‐procedural complications | 95 | 11 (11.6) |
| Any 24‐h ICHd | 81 | 11 (13.6) |
| 90‐day good outcome (mRS 0‐2) | 95 | 38 (40.0) |
| 90‐day mortality (mRS 6) | 95 | 30 (31.6) |
Values are n (%) or median (interquartile range) unless otherwise indicated. DWI pc‐ASPECTS indicates diffusion‐weighted imaging posterior‐circulation Alberta Stroke Program Early Computed Tomography Score; ICH, intracerebral hemorrhage; IVT, intravenous thrombolysis; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; mTICI, modified Treatment In Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale.
NIHSS score is defined as 42 in the setting of coma, locked‐in state, or tetraparesis.
Calculated among patients treated with IVT before endovascular treatment.
4 passes (n=7), 5 passes (n=4), 6 passes (n=1), and 8 passes (n=1).
Including 2 symptomatic ICH.
As shown in Table 2, the rate of 90‐day good outcome decreased with tertiles of onset to reperfusion time; however, the trend did not reach the significance level (P=0.16). With reperfusion time used as a continuous variable, the unadjusted OR per 30‐minute increase was 0.96 (95% CI 0.90‐1.02). In multivariate analysis adjusted for prespecified confounding factors (age, admission National Institutes of Health Stroke score, and reperfusion grade), the detrimental effect of longer onset to reperfusion time on good outcome was also not significant (adjusted OR 0.93; 95% CI 0.85‐1.01; P=0.074). Similar results were found when we analyzed the different interval times.
Table 2.
Association Between Different Time Intervals and 90‐Day Good Outcome (mRS 0‐2)
| Time Interval (Percentiles), min | OR (95% CI)a | P Value | |||
|---|---|---|---|---|---|
| <33rd | 33rd to 66th | >66th | |||
| Onset to reperfusion, min | 247 (190‐270)b | 368 (329‐405)b | 560 (491‐707)b | ||
| n (%) | 15/31 (48.4) | 13/32 (40.6) | 10/32 (31.3) | 0.16c | |
| Unadjusted OR (95% CI) | 1.00 (reference) | 0.73 (0.26‐1.98) | 0.48 (0.17‐1.36) | 0.96 (0.90‐1.02) | 0.23 |
| Adjusted OR (95% CI)d | 1.00 (reference) | 0.53 (0.14‐1.94) | 0.26 (0.05‐1.05) | 0.93 (0.85‐1.01) | 0.074 |
| Onset to imaging, min | 82 (65‐103)b | 137 (123‐147)b | 270 (231‐389)b | ||
| n (%) | 13/29 (44.8) | 12/30 (40.0) | 9/29 (31.0) | 0.28c | |
| Unadjusted OR (95% CI) | 1.00 (reference) | 0.82 (0.29‐2.31) | 0.55 (0.18‐1.62) | 0.96 (0.88‐1.05) | 0.35 |
| Adjusted OR (95% CI)d | 1.00 (reference) | 0.47 (0.12‐1.82) | 0.37 (0.09‐1.53) | 0.91 (0.81‐1.02) | 0.11 |
| Imaging to groin puncture, min | 73 (39‐89) | 142 (127‐153) | 225 (192‐302) | ||
| n (%) | 10/29 (34.5) | 13/30 (43.3) | 11/29 (37.9) | 0.79c | |
| Unadjusted OR (95% CI) | 1.00 (reference) | 1.45 (0.50‐4.17) | 1.16 (0.39‐3.40) | 1.02 (0.89‐1.16) | 0.82 |
| Adjusted OR (95% CI)d | 1.00 (reference) | 0.88 (0.22‐3.54) | 0.87 (0.22‐3.37) | 0.99 (0.85‐1.17) | 0.97 |
| Imaging to reperfusion, min | 126 (100 to 144)b | 204 (180‐217)b | 314 (277‐402)b | ||
| n (%) | 13/29 (44.8) | 11/30 (36.7) | 10/29 (34.5) | 0.42c | |
| Unadjusted OR (95% CI) | 1.00 (reference) | 0.71 (0.25‐2.02) | 0.65 (0.22‐1.87) | 0.96 (0.86‐1.07) | 0.49 |
| Adjusted OR (95% CI) d | 1.00 (reference) | 0.46 (0.11‐1.85) | 0.39 (0.09‐1.56) | 0.93 (0.80‐1.08) | 0.34 |
IQR indicates interquartile range; mRS, modified Rankin Scale; mTICI, modified Treatment In Cerebral Infarction; NIHSS, National Institute of Health Stroke score; OR, odds ratio.
Odds ratio calculated per 30‐min increase.
Median (IQR) of time interval.
Calculated using Cochran‐Armitage trend test.
Prespecified adjustment for reperfusion grade (mTICI 2b vs 3), age, and baseline NIHSS score.
When patients with determined DWI pc‐ASPECTS at baseline were divided into 2 groups according to the DWI pc‐ASPECTS cutoff value of 8, we found a significant heterogeneity in the association of good outcome with time between MRI and reperfusion (adjusted P‐value for heterogeneity=0.02). As shown in Table 3 and Figure, we observed a strong detrimental effect of MRI‐to‐reperfusion time in patients with DWI pc‐ASPECTS <8 (good outcome 39.4%; adjusted OR 0.40; 95% CI 0.18‐0.85), whereas no time effect was found in patients with DWI pc‐ASPECTS ≥8 (good outcome 53.6%; adjusted OR 1.10; 95% CI, 0.79‐1.51). Similar results were observed when the time between MRI and groin puncture was analyzed, with an adjusted OR 0.48 (95% CI 0.21‐1.07) in patients with DWI pc‐ASPECTS <8 and 1.23 (0.83‐1.80) in patients with DWI pc‐ASPECTS ≥8 (P for heterogeneity=0.045). No such heterogeneity was found with time between symptom onset and reperfusion or between symptom onset and MRI (Table 3).
Table 3.
Impact of Different Time Intervals on 90‐Day Good Outcome (mRS 0‐2) With Successful Reperfusion (mTICI 2b‐3) After Thrombectomy According to Baseline DWI pc‐ASPECTS
| DWI pc‐ASPECTS <8 (n=33) | DWI pc‐ASPECTS ≥8 (n=28) | P Value for Heterogeneity | |
|---|---|---|---|
| Good outcome (90‐day mRS≤2), n (%) | 13 (39.4) | 15 (53.6) | … |
| Symptoms to reperfusion, min | 374 (265‐505)a | 371 (311‐461)a | … |
| Unadjusted OR (95% CI) | 0.90 (0.79‐1.03) | 0.99 (0.88‐1.11) | 0.28 |
| Adjusted OR (95% CI)b | 0.87 (0.73‐1.02) | 0.93 (0.81‐1.06) | 0.46 |
| Symptoms to imaging, min | 121 (102‐224)a | 145 (107‐190)a | … |
| Unadjusted OR (95% CI) | 0.99 (0.88‐1.11) | 0.96 (0.84‐1.10) | 0.72 |
| Adjusted OR (95% CI)b | 0.99 (0.82‐1.19) | 0.88 (0.74‐1.03) | 0.34 |
| Imaging to groin puncture, min | 140 (107‐176) | 157 (121‐210) | … |
| Unadjusted OR (95% CI) | 0.66 (0.42‐1.04) | 1.19 (0.86‐1.64) | 0.037 |
| Adjusted OR (95% CI)b | 0.48 (0.21‐1.07) | 1.23 (0.83‐1.80) | 0.045 |
| Imaging to reperfusion, min | 194 (169‐291)a | 237 (167‐283)a | … |
| Unadjusted OR (95% CI) | 0.61 (0.39‐0.94) | 1.06 (0.80‐1.41) | 0.032 |
| Adjusted OR (95% CI)b | 0.40 (0.18‐0.85) | 1.10 (0.79‐1.51) | 0.020 |
Odds ratios were calculated per 30‐min increase. DWI pc‐ASPECTS indicates diffusion‐weighted imaging posterior‐circulation Alberta Stroke Program Early Computed Tomography Score; IQR, interquartile range; mRS, modified Rankin Scale; mTICI, modified Treatment In Cerebral Infarction; OR, odds ratio.
Median (IQR).
Adjusted for the following prespecified confounders: reperfusion grade (mTICI 2b vs 3), age, and admission NIHSS.
Figure 1.
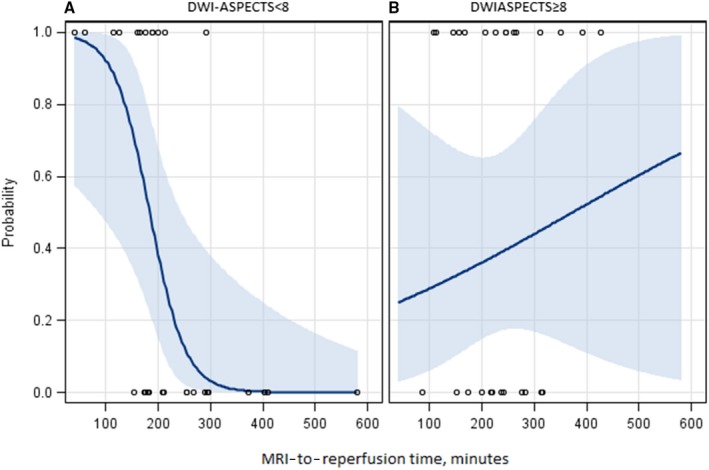
Predicted probability of good outcome (90‐day mRS 0‐22) according to imaging‐to‐reperfusion time in subgroups. Patients with DWI pc‐ASPECTS <8 (A) and DWI pc‐ASPECTS ≥8 (B) at baseline MRI. DWI pc‐ASPECTS indicates diffusion‐weighted imaging posterior‐circulation Alberta Stroke Program Early Computed Tomography Score; MRI, magnetic resonance imaging; mRS, modified Rankin Scale.
Discussion
Our main finding is that imaging‐to‐reperfusion time appears to be a crucial factor impacting the 90‐day clinical outcome in patients with acute BAO. In fact, in the setting of extensive baseline ischemia (defined as a baseline DWI pc‐ASPECTS <8), a functionally independent outcome may be achieved after rapid successful endovascular reperfusion. Thus, our data confirm the evidence for an aggressive target time metric in endovascular therapy for posterior circulation stroke patients, as reported in anterior circulation large‐vessel strokes.17 In the ESCAPE (Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke) trial, the median time from imaging to groin puncture was 51 (IQR 39‐68) minutes,17 whereas it was 141 (IQR 90‐188) minutes in our study. However, the median time from imaging to groin puncture was 73 (IQR 34‐124) minutes in patients directly admitted to a comprehensive stroke center. Our imaging‐to–groin puncture times appear too long and were mainly due to the high number of secondary transfer patients (n=73/93, 78.5%).
To date, few predictors of clinical outcome such as baseline National Institutes of Health Stroke score, smoking, and reperfusion status have been identified after reperfusion therapy with thrombolysis and/or thrombectomy in patients with acute BAO.7, 8, 9, 16, 18 The effect of time to treatment remains unclear in this subset of stroke patients. The BASICS study emphasized the impact of time with a significant increase in poor outcome when reperfusion occurred beyond 6 hours after symptom onset.4, 19 We also observed an association among symptom onset and reperfusion and outcome in the present study. However, it was not significant after adjustment, probably due to the small sample size. In contrast, whatever the baseline DWI pc‐ASPECTS, we found no influence of time between symptoms and imaging on outcome in our overall population. However, we can hypothesize that the included patients may present widely different ischemic consequences of BAO according to the thrombus location, angioarchitecture of the posterior circulation,20 quality of the reperfusion, and collateral circulation.20, 21 These factors, more than the time metric, may greatly influence the clinical presentation at admission, ischemic volume, potential benefit of the reperfusion, and therefore the clinical outcome. A parallel can be drawn with the anterior circulation where a major thrombectomy benefit has been recently demonstrated in some patients maintaining a low ischemic volume despite a large artery occlusion, far beyond 6 hours.22, 23
In this study we observed that, whatever the time from onset to admission, the key point was at imaging, with the characterization of the ischemic lesion extent, as illustrated in the anterior circulation strokes.17 There was a dramatic decrease in good outcome probability with the lengthening of time to reperfusion for patients with larger infarctions leading to a pc‐ASPECTS <8 at baseline DWI. These “fast‐progressor” patients appeared to benefit the least from compensatory mechanisms and should thus be considered as those for whom time from imaging to reperfusion is the most critical. Similar data were reported in the absence of extensive baseline ischemia, in which intravenous thrombolysis alone led to a good outcome in 50% of patients up to 48 hours.16 In addition, we also reported previously that DWI lesions may not always correspond to the core infarct and can be reversible, especially if complete recanalization is achieved early.23, 24
Our study presents some limitations, mainly due to the reduced size of the population studied. This could partially explain why we failed to demonstrate any impact of time from onset to reperfusion on outcome in the overall population. Further studies are required to confirm the major impact of imaging‐to‐reperfusion time in patients with posterior circulation occlusion treated by thrombectomy.
In conclusion, among acute stroke patients with BAO treated with modern endovascular thrombectomy, time from imaging to reperfusion impacts clinical outcome, highlighting that in‐hospital workflow management of patients with BAO should also be aggressive considering the target time metric, as for large‐vessel occlusions of the anterior circulation. Patients with low pc‐ASPECTS on baseline DWI should not be excluded from endovascular therapy because a good outcome may be achieved in the setting of a rapid reperfusion after imaging.
Disclosures
None.
The ETIS (Endovascular Treatment in Ischemic Stroke) Investigators
Jean‐Pierre Decroix, Adrien Wang, Serge Evrard, Maya Tchikviladzé, Frederic Bourdain, Jaime Gonzalez‐Valcarcel, Georges Rodesh, Oguzhan Coskun, Federico Di Maria, Fernando Pico, Haja Rakotoharinandrasana, Philippe Tassan, Roxanna Poll, Ovide Corabianu, Thomas de Broucker, Didier Smadja, Hocine Redjem, Gabriele Ciccio, Stanislas Smadja, Sonia Alamowitch, Robert Fahed, Jean‐Philippe Desilles, Mickael Obadia, Matthieu Fisselier, Candice Sabben, Olivier Ille, Eric Manchon, Pierre‐Yves Garcia, Francis Turjman, Roberto Riva, Paul‐Emile Labeyrie, Serge Bracard, René Anxionnat, Marc Braun, Emmanuelle Schmitt, Sophie Planel, Anne‐Laure Derelle, Romain Tonnelet, Liang Liao.
(J Am Heart Assoc. 2019;8:e010962. DOI: 10.1161/JAHA.118.010962.)
Contributor Information
Morgan Guillaume, Email: mgpro21@gmail.com.
the Endovascular Treatment in Ischemic Stroke (ETIS) Investigators:
Jean‐Pierre Decroix, Adrien Wang, Serge Evrard, Maya Tchikviladzé, Frederic Bourdain, Georges Rodesh, Oguzhan Coskun, Federico Di Maria, Fernando Pico, Haja Rakotoharinandrasana, Philippe Tassan, Roxanna Poll, Ovide Corabianu, Thomas de Broucker, Didier Smadja, Hocine Redjem, Gabriele Ciccio, Stanislas Smadja, Sonia Alamowitch, Robert Fahed, Jean‐Philippe Desilles, Mickael Obadia, Matthieu Fisselier, Candice Sabben, Olivier Ille, Eric Manchon, Pierre‐Yves Garcia, Francis Turjman, Roberto Riva, Paul‐Emile Labeyrie, Serge Bracard, René Anxionnat, Marc Braun, Emmanuelle Schmitt, Sophie Planel, Anne‐Laure Derelle, Romain Tonnelet, and Liang Liao
References
- 1. Gory B, Turjman F. Thrombectomy after intravenous thrombolysis is the new standard of care in acute stroke with large vessel occlusion. Interv Neuroradiol. 2015;21:691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu X, Xu G, Liu Y, Zhu W, Ma M, Xiong Y, Zi W, Dai Q, Leung T, Yan B, Davis S, Liebeskind DS, Pereira VM, Nogueira RG; BEST Trial Investigators . Acute basilar artery occlusion: endovascular interventions versus standard medical treatment (BEST) trial‐design and protocol for a randomized, controlled, multicenter study. Int J Stroke. 2017;12:779–785. [DOI] [PubMed] [Google Scholar]
- 3. Van der Hoeven EJ, Schonewille WJ, Vos JA, Algra A, Audebert HJ, Berge E, Ciccone A, Mazighi M, Michel P, Muir KW, Obach V, Puetz V, Wijman CA, Zini A, Kappelle JL; BASICS Study Group . The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. 2013; 14:200 DOI: 10.1186/1745-6215-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schonewille WJ, Wijman CAC, Michel P, Rueckert CM, Weimar C, Mattle HP, Engelter ST, Tanne D, Muir KW, Molina CA, Thijs V, Audebert H, Pfefferkorn T, Szabo K, Lindsberg PJ, de Freitas G, Kappelle LJ, Algra A; BASICS Study Group . Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8:724–730. [DOI] [PubMed] [Google Scholar]
- 5. Nagel S, Kellert L, Möhlenbruch M, Bösel J, Rohde S, Ringleb P. Improved clinical outcome after acute basilar artery occlusion since the introduction of endovascular thrombectomy devices. Cerebrovasc Dis. 2013;36:394–400. [DOI] [PubMed] [Google Scholar]
- 6. Gory B, Eldesouky I, Sivan‐Hoffmann R, Rabilloud M, Ong E, Riva R, Gherasim DN, Turjman A, Nighoghossian N, Turjman F. Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry. 2016;87:520–525. [DOI] [PubMed] [Google Scholar]
- 7. Gory B, Mazighi M, Labreuche J, Blanc R, Piotin M, Turjman F, Lapergue B; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators . Predictors for mortality after mechanical thrombectomy of acute basilar artery occlusion. Cerebrovasc Dis. 2018;45:61–67. [DOI] [PubMed] [Google Scholar]
- 8. Kang DH, Jung C, Yoon W, Kim SK, Baek BH, Kim JT, Park MS, Kim YW, Hwang YH, Kim YS, Kim BJ, Han MK, Bae HJ. Endovascular thrombectomy for acute basilar artery occlusion: a multicenter retrospective observational study. J Am Heart Assoc. 2018;7:7–14. DOI: 10.1161/JAHA.118.009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoon W, Kim SK, Heo TW, Baek BH, Lee YY, Kang HK. Predictors of good outcome after stent‐retriever thrombectomy in acute basilar artery occlusion. Stroke. 2015;46:2972–2975. [DOI] [PubMed] [Google Scholar]
- 10. Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, De Miquel MA, Millan M, Castaño C, Urra X, Sanroman L, Dàvalos A, Jovin T; for the REVASCAT Trial Investigators . Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47:999–1004. [DOI] [PubMed] [Google Scholar]
- 11. Gory B, Mazighi M, Blanc R, Labreuche J, Piotin M, Turjman F, Lapergue B; Endovascular Treatment in Ischemic Stroke (ETIS) Research Investigators . Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first‐line strategy (ADAPT vs stent retriever). J Neurosurg. 2018;12:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, TOAST Investigators . Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, Becker U, Urban G, O'Reilly C, Barber PA, Sharma P, Goyal M, Gahn G, von Kummer R, Demchuk AM. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. [DOI] [PubMed] [Google Scholar]
- 14. Nagel S, Herweh C, Köhrmann M, Huttner HB, Poli S, Hartmann M, Hähnel S, Steiner T, Ringleb P, Hacke W. MRI in patients with acute basilar artery occlusion—DWI lesion scoring is an independent predictor of outcome. Int J Stroke. 2012;7:282–288. [DOI] [PubMed] [Google Scholar]
- 15. Dennis M, Mead G, Doubal F, Graham C. Determining the modified Rankin score after stroke by postal and telephone questionnaires. Stroke. 2012;43:851–853. [DOI] [PubMed] [Google Scholar]
- 16. Strbian D, Sairanen T, Silvennoinen H, Salonen O, Kaste M, Lindsberg PJ. Thrombolysis of basilar artery occlusion: impact of baseline ischemia and time. Ann Neurol. 2013;73:688–694. [DOI] [PubMed] [Google Scholar]
- 17. Menon BK, Sajobi TT, Zhang Y, Rempel JL, Shuaib A, Thornton J, Williams D, Roy D, Poppe AY, Jovin TG, Sapkota B, Baxter BW, Krings T, Silver FL, Frei DF, Fanale C, Tampieri D, Teitelbaum J, Lum C, Dowlatshahi D, Eesa M, Lowerison MW, Kamal NR, Demchuk AM, Hill MD, Goyal M. Analysis of workflow and time to treatment on thrombectomy outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) randomized, controlled trial. Circulation. 2016;133:2279–2286. [DOI] [PubMed] [Google Scholar]
- 18. Bouslama M, Haussen CD, Aghaebrahim A, Grossberg JA, Walker G, Rangaraju S, Horev A, Frankel MR, Nogueira RG, Jovin TG, Jadhav AP. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke. 2017;48:3252–3257. [DOI] [PubMed] [Google Scholar]
- 19. Vergouwen MDI, Algra A, Pfefferkorn T, Weimar C, Rueckert CM, Thijs V, Kappelle LJ, Schonewille WJ; Artery International Cooperation Study (BASICS) Study Group . Time is brain(stem) in basilar artery occlusion. Stroke. 2012;43:3003–3006. [DOI] [PubMed] [Google Scholar]
- 20. Maus V, Kalkan A, Kabbasch C, Abdullayev N, Stetefeld H, Barnikol UB, Liebig T, Dohmen C, Fink GR, Borggrefe J, Mpotsaris A. Mechanical thrombectomy in basilar artery occlusion: presence of bilateral posterior communicating arteries is a predictor of favorable clinical outcome. Clin Neuroradiol. 2017. DOI: 10.1007/s00062-017-0651-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Singer OC, Berkefeld J, Nolte CH, Bohner G, Haring HP, Trenkler J, Gröschel K, Müller‐Forell W, Niederkorn K, Deutschmann H, Neumann‐Haefelin T, Hohmann C, Bussmeyer M, Mpotsaris A, Stoll A, Bormann A, Brenck J, Schlamann MU, Jander S, Turowski B, Petzold GC, Urbach H, Liebeskind DS; ENDOSTROKE Study Group . Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol. 2015;77:415–424. [DOI] [PubMed] [Google Scholar]
- 22. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 23. Gory B, Ritzenthaler T, Riva R, Nighoghossian N, Turjman F. Reversibility of brainstem damage after a mechanical thrombectomy. JAMA Neurol. 2014;71:646–647. [DOI] [PubMed] [Google Scholar]
- 24. Gory B, Sivan‐Hoffmann R, Riva R, Labeyrie PE, Eldesouky I, Sadeh‐Gonike U, Signorelli F, Turjman F. DWI lesions reversal in posterior circulation stroke after reperfusion: two illustrative cases and review of the literature. J Neuroradiol. 2015;42:184–187. [DOI] [PubMed] [Google Scholar]


