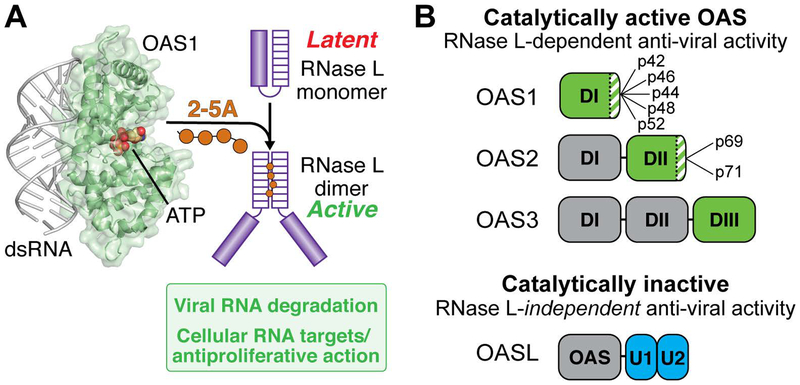
Figure 1. Overview of the OAS/RNase L pathway.
A. dsRNA binding to OAS (here shown as OAS1) promotes synthesis of 2’−5’-linked oligoadenylates (2–5A) which in turn induce dimerization and activation of the latent ribonuclease (RNase L). RNase L degrades viral and cellular RNA targets to promote antiviral responses. B. Summary of domain organization and activities of the four human OAS proteins: OAS1, OAS2, OAS3, and OASL. For the active OAS proteins (OAS1, OAS2, and OAS3), the domain responsible for 2–5A catalysis is shown in green; other OAS domains (grey) contribute to dsRNA binding. Splicing isoforms are indicated for OAS1 and OAS2 (OAS3 is expressed as a single isoform); these proteins differ in their C-terminus (green/white striped region). Human OASL contains a single non-catalytically active OAS domain appended with tandem ubiquitin-like domains (U1/U2; blue) and functions in antiviral innate immunity independent of RNase L.