FIGURE 1.
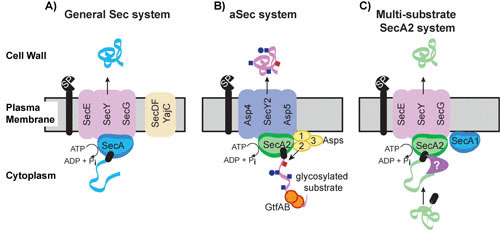
Models for the general Sec system, the aSec system, and the multisubstrate SecA2 system. (A) General Sec system. SecA uses ATP hydrolysis to export cytoplasmic preproteins through the SecYEG channel in an unfolded state. SecDFYajC are auxiliary components that enhance export efficiency. Sec signal peptides (black rectangle) target preproteins (blue ribbon) for export through SecYEG. Following export across the membrane, the signal peptide is cleaved by a signal peptidase (SP) and the resulting mature protein folds into its proper conformation. (B) aSec system. The model depicted is largely based on studies of the S. gordonii SecA2 system. Glycosylation of the preprotein (pink ribbon) with GlcNAc (blue squares) and Glc (blue circles) likely occurs cotranslationally. The positively charged N region of the signal peptide (black rectangle) targets the preprotein to anionic phospholipids, which aids the localization with SecA2. Transport through the SecY2/Asp4/5 channel requires a specific sequence in the mature region of the preprotein, as well as Asp1 to Asp3. Asp2 is a bifunctional protein that also mediates O-acetylation of GlcNAc moieties (red square). Cleavage of the signal peptide is thought to be carried out by the general SP. (C) Multisubstrate SecA2 system. The model depicted is largely based on studies of the mycobacterial SecA2 system. SecA2 works with the canonical SecYEG channel and possibly SecA1 to export its specific subset of preproteins (green ribbon). The majority of SecA2 substrates are synthesized as preproteins with a signal peptide (black rectangle) that is cleaved in association with export. The mature domain, not the signal peptide, of a preprotein determines if a protein is exported by this SecA2 system. It is proposed that the mature domain of a SecA2 substrate has the propensity to fold in the cytoplasm and that the role of SecA2 is to facilitate the export of such proteins, in an unfolded state, through the SecYEG channel. Additional factors are likely to work with SecA2 in the pathway (purple symbol). The role of SecA2 in exporting moonlighting proteins that lack signal peptides is unclear and not depicted in the model.
