Summary
First‐line treatments for classical Hodgkin lymphoma (HL) include ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) and BEACOPP escalated (escalated dose bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone). To further improve overall outcomes, positron emission tomography‐driven strategies and ABVD or BEACOPP variants incorporating the antibody‐drug conjugate brentuximab vedotin (BV) or anti‐PD1 antibodies are under investigation in advanced‐stage patients. The present study aimed to elicit preferences for attributes associated with ABVD, BEACOPP escalated and BV‐AVD (BV, adriamycin, vinblastine and dacarbazine) among patients and physicians. Cross‐sectional online discrete choice experiments were administered to HL patients (n = 381) and haematologists/oncologists (n = 357) in France, Germany and the United Kingdom. Included attributes were progression‐free survival (PFS), overall survival (OS), and the risk of neuropathy, lung damage, infertility and hospitalisation due to adverse events. Whereas 5‐year PFS and OS were the most important treatment attributes to patients, the relative importance of each attribute and preference weights for each level varied among physicians according to the description of the hypothetical patient for whom treatment was recommended. PFS and OS most strongly influenced physicians’ recommendations when considering young female patients who did not want children or young male patients. Infertility was more important to physicians’ treatment decision than PFS when considering young women with unknown fertility preferences, whereas hospitalisations due to adverse events played the largest role in treatment decisions for older patients.
Keywords: Hodgkin lymphoma, patient and physician preferences, discrete choice experiment, ABVD, BEACOPP
Hodgkin lymphoma (HL) is a cancer of the lymphatic system, originating from lymphocyte blood cells. Classical HL, identified by CD30‐positive malignant Hodgkin and Reed‐Sternberg (HRS) cells, is by far the most common histology, accounting for approximately 95% of HL cases (Eichenauer et al, 2018). The incidence of HL is slightly higher in males and has a bimodal age distribution, occurring most frequently between 20–35 and after 55 years of age (SEER, 2016). Based on the disease extent by imaging, stages I to IV might be present according to the modified Ann Arbor classification. Taking into account stage and risk factors, early‐stage favourable, early‐stage unfavourable and advanced‐stage disease are differentiated for the purpose of treatment allocation.
For individuals with newly diagnosed advanced‐stage HL (stage III/IV or stage IIB with large mediastinal mass and/or extranodal disease), multimodal chemotherapy is considered standard of care. Radiotherapy is recommended for patients who have evidence of localized residual disease after the end of systemic chemotherapy (Eichenauer et al, 2018). The standard treatment regimens for advanced‐stage HL included in the current European Society for Medical Onclogy (ESMO) guidelines are adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) and escalated dose bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone (BEACOPPescalated); the latter is an established standard for younger patients and recommended only for those up to the age of 60 (Eichenauer et al, 2018).
The optimal choice of first‐line treatment for advanced‐stage HL (ABVD or BEACOPPescalated) remains controversial amongst treating physicians, because despite the superiority of BEACOPPescalated in response rates and progression‐free survival (PFS), it has an increased toxicity profile. A randomized trial comparing 6 cycles ABVD with 4 cycles BEACOPPescalated + 2 cycles BEACOPPbaseline in patients with advanced‐stage HL reported a significantly improved 5‐year PFS rate for BEACOPP of 81% compared to 68% for ABVD (P = 0·038) and a non‐significant difference in 5‐year overall survival (OS) rates (92% vs. 84%)(Federico et al, 2009). However, a more recent network meta‐analysis that included a total of 14 clinical trials of treatments for advanced HL indicated that BEACOPPescalated is associated with a significantly longer 5‐year OS than ABVD (95% vs. 88%) (Skoetz et al, 2013). More recent positron emission tomography (PET)‐adapted strategies not directly comparing ABVD and BEACOPPescalated were evaluated within large‐scale randomized phase III trials. After an initial 2 cycles of ABVD, a 3‐year PFS of 84·4% and 85·7% was reported for PET‐negative patients continuing treatment with 4 additional cycles of AVD (adriamycin, vinblastine and dacarbazine) and ABVD, respectively. In PET‐positive patients, 3‐year PFS was 67·5% after an additional 4 cycles of BEACOPPescalated (Johnson et al, 2016). For patients who were PET‐negative and ‐positive, respectively, after 2 cycles of BEACOPPescalated, a 5‐year PFS of 92·2% and 89·7% was reported with 2 or 4 additional cycles of BEACOPPescalated (Borchmann et al, 2018).
Adverse effects of treatments are common, and include organ damage (Bhakta et al, 2016), sterility (Behringer et al, 2013), reduced long‐term quality of life (QoL), e.g. due to fatigue (Kreissl et al, 2016), and increased risk of secondary cancers (Schaapveld et al, 2015). However, the risks and types of side effects experienced differ between ABVD and BEACOPPescalated, with notably more acute side effects due to haematological toxicity with BEACOPPescalated (Federico et al, 2009).
To reduce short‐ and long‐term toxicity of BEACOPPescalated or improve efficacy of ABVD, variants of the regimens incorporating novel agents, such as the anti‐CD30 antibody‐drug conjugate brentuximab vedotin (BV) or the anti‐PD1 antibody nivolumab, are under investigation. Results of the randomized phase III ECHELON‐1 trial with BV‐AVD versus ABVD and a phase II trial investigating BV‐based BEACOPP variants were recently reported (Ansell et al, 2014; Connors et al, 2017; Eichenauer et al, 2017).
With various treatment options with different outcomes/adverse event risk profiles already approved or under investigation, physician and patient preferences with regards to potential toxicities and treatments are important when making treatment decisions. The importance of the patient voice and a patient‐centric approach to care and treatment has been increasingly recognized over the last years, as exemplified by the Food and Drug Administration (FDA)'s Patient Preference Initiative FDA, 2017) and the efforts of the European Medicines Agency (EMA) to integrate the patient voice into medicine evaluations (EMA, 2013). In particular, a broad understanding of HL patients’ preferences and the trade‐offs they would be willing to make can be important for treating physicians regarding the balance of curing the malignancy and minimizing the risks of acute and late toxicities. Additionally, it is valuable to understand physicians’ preferences for attributes of HL treatments, in order to understand treatment patterns and potential differences in treatment attribute priorities and trade‐offs between physicians and patients. The objective of this study was to elicit preferences for attributes and attribute levels associated with first‐line treatments for advanced‐stage HL among patients and treating physicians in France, Germany and the United Kingdom (UK). The first‐line treatments considered in this study when selecting attributes and levels included ABVD, BEACOPPescalated and BV‐AVD.
Methods
Cross‐sectional online surveys including a discrete choice experiment (DCE) were administered to patients and physicians in France, Germany and the UK. The study plan was reviewed and approved by the Western Institutional Review Board (WIRB) prior to data collection.
Participants
Individuals diagnosed with HL who were about to undergo first‐line HL treatment or had undergone treatment within the previous 2 years were eligible for the patient survey. While the objectives are specific to treatment of advanced‐stage HL, patients with self‐reported early‐stage HL were not excluded to ensure that an adequate sample size could be achieved. Physicians who specialized in haematological malignancies and had treated a patient with HL within the previous 2 years were eligible for the physician survey.
Survey development and data collection
The development of the surveys, specifically the selection of the attributes and levels included in the DCE component of the surveys, were informed from a targeted literature review, clinical expert interviews and patient qualitative interviews. The targeted literature review helped identify potential attributes of HL chemotherapies that may impact preferences and determine which attributes differ between treatments of interest. One clinical expert from each target country was interviewed to further understand the importance of potential attributes from the physician perspective and obtain feedback on the attribute and level wording for both the patient and physician surveys. Ten individuals diagnosed with HL from the UK participated in qualitative interviews to understand the patient perspective regarding the attribute importance and obtain feedback on the attribute and level wording for the patient survey. Patients for the qualitative interviews were recruited through support groups and nurse and consumer networks. Patient interviews were conducted in‐person following a semi‐structured interview guide. All patients provided informed consent prior to participating in the interview. A manageable number of attributes that were identified as being key to the decision‐making process from the patient and physician perspective were selected. Attribute levels also needed to differ between first‐line HL treatments and long‐term estimates for survival needed to be available to populate the levels.
The target sample size for the surveys was 100 patients and 100 physicians with logical responses per country. Participants were recruited from a research database of patients and physicians [Medefield Ltd, New York, NY (www.medefield.com/)]. The survey was pilot tested in English among a small sample of physicians and patients. The final survey was administered in the native language for each country. All participants provided consent prior to completing the survey and were compensated for their time.
The patient survey included questions on demographics, clinical characteristics and treatment history. The physician survey included questions regarding demographics, clinical experience and practice setting.
The DCE presented treatment profiles that included six attributes, each with two to three levels. Levels were populated based on a targeted literature review (keyword searches in Pubmed Central in July 2016) of studies using the treatments ABVD or BEACOPPescalated in stage III and IV HL (Federico et al, 2009; Mounier et al, 2014; Carde et al, 2016), as well as on estimates based on anticipated outcomes of the ECHELON‐1 trial for BV‐AVD [NCT01712490] and interim read‐outs and information from a phase I study that had used BV‐AVD (Younes et al, 2013). The attributes and levels included: 5‐year OS (levels: 84%, 89%, 94%); 5‐year PFS (levels: 68%, 77%, 85%); Risk of side effects requiring emergency room or hospital visit (levels: 40%, 80%); Risk of peripheral neuropathy (levels: low risk, high risk); Risk of infertility (levels: low risk, high risk); and Risk of permanent pulmonary toxicity (levels: no risk, 20% risk).
Based on patient interviews, the PFS attribute was presented differently in the patient DCE as the risk of HL coming back or getting worse within 5‐year (levels: 26%, 15%, 4%). These values were calculated by subtracting PFS from OS in a way that created the largest possible and smallest possible values, in order to cover the full range possible.
Participants each reviewed 12 unique DCE scenarios, developed using a d‐efficient design, and selected their preference between two hypothetical unnamed treatments. Patients considered themselves when selecting their preference and were given the option to select neither treatment (opt‐out option). Physicians were asked to consider five different advanced‐stage HL patient types that differed in gender, age (30 vs. 65 years), smoking status and desire to have children. Physicians were not given an opt‐out option.
The DCE also included a dominant scenario test, to check for logical responses, as well as a repeat scenario test to assess consistency between responses. The dominant scenario presented one treatment option that was superior in all attributes to the other treatment option, i.e. it had longer OS and PFS as well as a lower risk of all side effects. Participants who chose the treatment option that was inferior in all respects were considered to have failed the dominant scenario logic test. In addition, a check for consistency was included, where patients were presented with the same comparison twice at different points of the survey. An answer was considered inconsistent if the patient did not choose the same treatment as preferred in both scenarios. Inconsistent answers may be an effect of learning; however, a failure to identify a dominant scenario indicates that the participant may have struggled with the complexity of the task or not have paid sufficient attention.
Analysis
The primary analysis was conducted among participants that had a logical response for the dominant scenario test. Inconsistent answers were not excluded from the main analysis, as they may be the result of a learning effect rather than a mistake. Two sensitivity analyses were conducted: One analysis included all participants, whether or not they failed the logic test, and the other one included only participants who had passed the logic test and shown consistency in their answers.
The DCE data were analysed using a mixed logit model (MXL, analysed in R version 3.3.3; https://cran.r-project.org/bin/windows/base/old/3.3.3/) in order to account for preference heterogeneity, which produces mean preference weight of each attribute level along with a standard deviation of effects. The model investigated main effects only. The OS and PFS attributes were treated as continuous variables in the model after demonstrating linearity. Dummy coding was used for the remaining categorical variables. Separate models were run for each of the five patient types considered by physicians and one model was run for the patient data. The relative importance of each attribute was calculated by determining the difference between the minimum and maximum coefficients of each attribute. These are presented as percentages, which can be interpreted as the weight of each attribute on the physician or patient treatment decision. The relative importance of the attributes is specific to the ranges included in the DCE. The relative importance of attributes from the patient perspective was also analysed according to the following patient subgroups: age (<55 years; ≥55 years), gender, disease stage [early ‐stage (defined as stage I, stage II, early); intermediate/advanced‐stage [defined as stage III, stage IV, intermediate, advanced)], treatment status and, for those patients who had completed treatment, remission status.
Results
Patient preferences
A total of 381 patients completed the survey. Approximately 80% of French and German and 65% of UK patients had logical responses to the dominant scenario, for a total of 289 patients included in the primary analysis (France = 102; Germany = 102; UK = 85). Among patients in the primary analysis, approximately two‐thirds were male (63%) and their median age was 36 years (range: 19–75 years; Table 1). The mean time since diagnosis was 21 months, ranging from 19 months among participants in the UK to 23 months among patients in Germany. Patients reported most frequently that they had been diagnosed in stage I (23%) and stage II (20%) across all regions, with a further 7·7% diagnosed in stages III or IV. Considering risk groups, a higher proportion of patients reported having been diagnosed with intermediate‐stage HL across all regions (28%), and a minority reported having been diagnosed in advanced‐stage (2·8%); 9% of patients could not recall their staging at diagnosis (Table 2). Among patients in the primary analysis set, 41% were currently on treatment for HL at the time of the survey, 29% had completed treatment and 25% had made a treatment decision but treatment had not yet started, and 5% had not yet made a decision. Thus, a total of 70% of the patients included in the analysis has experience of HL treatment and, potentially, the associated side effects. Among this subset of patients, the mean time from initiation of first‐line treatment was 1·2 years. Most patients who completed treatment reported that they attained a complete response (75%), while 28% reported relapsed disease.
Table 1.
Patient demographic characteristics (self‐reported)
| Total (N = 289) | France (N = 102) | Germany (N = 102) | UK (N = 85) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age (years) | ||||
| Mean (SD) | 36·6 (11·3) | 37·4 (11·9) | 37·5 (11·7) | 34·6 (9·7) |
| Median (range) | 36 (19–75) | 37 (20–72) | 37 (19–69) | 34 (19–75) |
| Sex | ||||
| Males | 181 (62·6) | 68 (66·7) | 62 (60·8) | 51 (60·0) |
| Females | 105 (36·3) | 33 (32·4) | 38 (37·3) | 34 (40·0) |
| Did not disclose | 3 (1·0) | 1 (1·0) | 2 (2·0) | 0 (0·0) |
| Education | ||||
| Less that high school or equivalent | 1 (0·3) | 0 (0·0) | 1 (1·0) | 0 (0·0) |
| High school or equivalent | 53 (18·3) | 12 (11·8) | 29 (28·4) | 12 (14·1) |
| Technical school/training | 55 (19·0) | 10 (9·8) | 36 (35·3) | 9 (10·6) |
| Some college, university or other post‐secondary education | 35 (12·1) | 18 (17·6) | 0 (0·0) | 17 (20·0) |
| College, university or other post‐secondary education | 106 (36·7) | 41 (40·2) | 35 (34·3) | 30 (35·3) |
| Graduate degree | 39 (13·5) | 21 (20·6) | 1 (1·0) | 17 (20·0) |
| Has dependents | 164 (56·7) | 57 (55·9) | 55 (53·9) | 52 (61·2) |
| Employment status | ||||
| Working full time | 168 (58·1) | 61 (59·8) | 62 (60·8) | 45 (52·9) |
| Working part time | 53 (18·3) | 19 (18·6) | 19 (18·6) | 15 (17·6) |
| Student | 3 (1·0) | 1 (1·0) | 0 (0·0) | 2 (2·4) |
| Retired | 12 (4·2) | 6 (5·9) | 3 (2·9) | 3 (3·5) |
| Homemaker | 8 (2·8) | 0 (0·0) | 2 (2·0) | 6 (7·1) |
| Not working due to health reasons other than HL | 11 (3·8) | 2 (2·0) | 3 (2·9) | 6 (7·1) |
| Not working due to HL | 32 (11·1) | 13 (12·7) | 12 (11·8) | 7 (8·2) |
| Not working due to other reasons | 2 (0·7) | 0 (0·0) | 1 (1·0) | 1 (1·2) |
| Other | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
HL, Hodgkin lymphoma; IQR, interquartile range; n, number; SD, standard deviation.
Table 2.
Patient treatment characteristics (self‐reported)
| All patients (n = 289) | |
|---|---|
| n (%) | |
| Treatment status | |
| No treatment decision made | 13 (4.5) |
| Decision made, not yet started treatment | 74 (25.6) |
| Currently on treatment | 119 (41.2) |
| Completed treatment | 83 (28.7) |
|
Patients who have started treatment (n = 202) |
|
| Time since initiation of first‐line HL treatment (years) | |
| Mean (SD) | 1.2 (0.7) |
| Median (IQR) | 1 (0‐2) |
|
Patients who have completed treatment (n = 83) |
|
| Response to front‐line | |
| Complete response/in remission | 62 (74.7) |
| Relapsed | 23 (27.7) |
| Not a complete response/not in remission | 15 (18.1) |
| Unknown | 6 (7.2) |
| Treatment following front‐line | |
| Radiotherapy | 23 (27.7) |
| Chemotherapy | 25 (30.1) |
| Immunotherapy/targeted therapy | 10 (12.0) |
| Stem cell transplant | 1 (1.2) |
| Additional treatment was unknown | 7 (8.4) |
| No additional treatment | 17 (20.5) |
| Recently experienced health issues | |
| Lung damage | 11 (13.3) |
| Heart damage | 2 (2.4) |
| Another cancer | 42 (50.6) |
| Peripheral neuropathy | 4 (4.8) |
| None | 32 (38.6) |
In the DCE part of the survey, a statistically significant preference for treatments with increased 5‐year OS outcomes, and decreased risk of 5‐year progression or relapse and short‐term side effects was identified for patients (P < 0·001; Fig 1). Additionally, patients significantly preferred treatments that offered a lower risk of peripheral neuropathy, infertility and pulmonary toxicity (P < 0·001). The magnitude of patient preferences for continuous variables was highest for increases in OS (mean preference weight: 0·056 per 1% increase in OS). Mean preference weights decreased by 0·033 per 1% increase in risk of relapse or progression and decreased by 0·006 per 1% increase in the risk of short‐term side effects. The preference for treatments associated with low risk of peripheral neuropathy, infertility and pulmonary toxicity were similar, where preference weights for the higher risk levels ranged from −0·484 to −0·501 (relative to the low risk or 0 risk level for each attribute). A total of 34 out of all 381 patients who completed the survey chose the opt‐out option for at least one DCE scenario.
Figure 1.
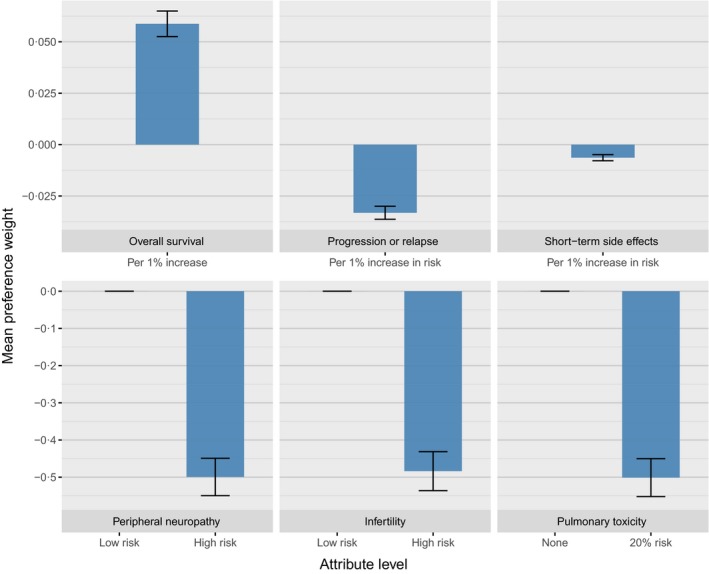
Mean preference weights for the patient discrete‐choice experiment. A positive weight indicates that preference weight increases as the level value increases (e.g. higher preference for higher overall survival). A negative weight indicates that the preference weight decreases as the level value increases (e.g. a lower preference for a higher risk of side effects). [Colour figure can be viewed at wileyonlinelibrary.com]
Physician preferences
A total of 357 physicians completed the survey. The proportion of physicians who had logical responses to the dominant scenario was similar across countries (78–82%) for a total of 281 physicians included in the primary analysis (France = 96; Germany = 92; UK = 93). Among physicians in the primary analysis, nearly three‐quarters were male (73%) and they reported a median of 15 years’ experience managing patients with HL (range: 1–35 years; Table 3). When asked about typical first‐line treatments used for patients with advanced HL, the majority of physicians in France and Germany selected ABVD, BEACOPPescalated and BEACOPP regimens as potential options. However, in the UK a clear preference for ABVD was observed, with 96% of physicians reporting this regimen as a typical first‐line treatment used.
Table 3.
Physician characteristics (self‐reported)
| Total (N = 281) | France (N = 96) | Germany (N = 92) | UK (N = 93) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Sex | ||||
| Males | 206 (73·3) | 73 (76·0) | 63 (68·5) | 70 (75·3) |
| Age (years) | ||||
| Median (IQR) | 47·0 (17–67) | 47·0 (31–67) | 46·0 (17–67) | 48·0 (33–61) |
| Specialty | ||||
| Haematologist | 102 (36·3) | 55 (57·3) | 11 (12·0) | 36 (38·7) |
| Oncologist | 18 (6·4) | 4 (4·2) | 8 (8·7) | 6 (6·5) |
| Haematologist‐oncologist | 161 (57·3) | 37 (38·5) | 73 (79·3) | 51 (54·8) |
| Practice setting | ||||
| Hospital‐based cancer centre | 178 (63·3) | 80 (83·3) | 23 (25·0) | 75 (80·6) |
| Academic‐based cancer centre | 94 (33·5) | 17 (17·7) | 50 (54·3) | 27 (29·0) |
| Private practice | 39 (13·9) | 3 (3·1) | 31 (33·7) | 5 (5·4) |
| Practice follows a guiding committee for HL treatment | ||||
| Deutsche/German Hodgkin studies | 116 (32·5) | 15 (12·2) | 95 (80·5) | 6 (5·2) |
| National Comprehensive cancer network | 97 (27·2) | 33 (26·8) | 32 (27·1) | 32 (27·6) |
| American Society of clinical oncology | 119 (33·3) | 57 (46·3) | 33 (28·0) | 29 (25·0) |
| European Society for medical oncology | 172 (48·2) | 80 (65·0) | 43 (36·4) | 49 (42·2) |
| European S3 guidelines | 60 (16·8) | 18 (14·6) | 38 (32·2) | 4 (3·4) |
| German multi‐centre ALL | 30 (8·4) | 4 (3·3) | 25 (21·2) | 1 (0·9) |
| Hospital board | 56 (15·7) | 30 (24·4) | 7 (5·9) | 19 (16·4) |
| Hospital formulary/guidelines | 95 (26·6) | 26 (21·1) | 10 (8·5) | 59 (50·9) |
| Other national/country specific guidelines | 65 (18·2) | 27 (22·0) | 3 (2·5) | 35 (30·2) |
| Other | 28 (7·8) | 10 (8·1) | 3 (2·5) | 15 (12·9) |
| Typical first line treatment regimens | ||||
| AVD | 99 (27·7) | 45 (36·6) | 23 (19·5) | 31 (26·7) |
| ABVD | 298 (83·5) | 104 (84·6) | 85 (72·0) | 109 (94·0) |
| BEACOPP | 201 (56·3) | 79 (64·2) | 74 (62·7) | 48 (41·4) |
| BEACOPPesc | 208 (58·3) | 89 (72·4) | 83 (70·3) | 36 (31·0) |
| Clinical trial | 18 (5·0) | 6 (4·9) | 5 (4·2) | 7 (6·0) |
| Knowledgeable of new regimens | ||||
| AVD + bevacizumab | 135 (37·8) | 45 (36·6) | 43 (36·4) | 47 (40·5) |
| AVD + brentuximab vedotin | 280 (78·4) | 104 (84·6) | 80 (67·8) | 96 (82·8) |
| AD + brentuximab vedotin | 156 (43·7) | 51 (41·5) | 54 (45·8) | 51 (44·0) |
| BrECADD | 171 (47·9) | 52 (42·3) | 79 (66·9) | 40 (34·5) |
ABVD, adriamycin, bleomycin, vinblastine and dacarbazine; AD, adriamycin and dacarbazine; ALL, acute lymphoblastic leukaemia; AVD, adriamycin, vinblastine and dacarbazine; BEACOPP, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone; BEACOPPesc, escalated dose bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone; BrECADD, brentuximab vedotin, etoposide, doxorubicin, cyclophosphamide, dacarbazine and dexamethasone; HL, Hodgkin lymphoma; IQR, interquartile range.
Across all five MXL models for each patient description, physicians expressed a statistically significant preference for treatments with higher 5‐year OS and PFS efficacy outcomes, and decreased risk of short‐term side effects (P < 0·0001), and significant preference for treatments that offer a low risk of peripheral neuropathy, infertility and pulmonary toxicity (P < 0·001; Fig 2). While the direction of preferences was the same across patient types, differences were observed in the magnitude of preferences. Most notably, when considering a patient profile of a 30‐year‐old woman with unknown fertility preferences, mean preference weights for the high risk infertility level was −1·132 compared to −0·438 when considering a profile of a 30‐year‐old woman who expressed no interest in having children and −0·328 when considering a profile of a 65‐year‐old woman. Notable differences in the magnitude of preference weights for the pulmonary toxicity attribute levels were also noted when physicians considered a profile of a female smoker compared to the profile of 30‐year‐old female non‐smoker (−1·668 vs. −0·450 for the 25% risk level relative to the 0% risk level, respectively).
Figure 2.
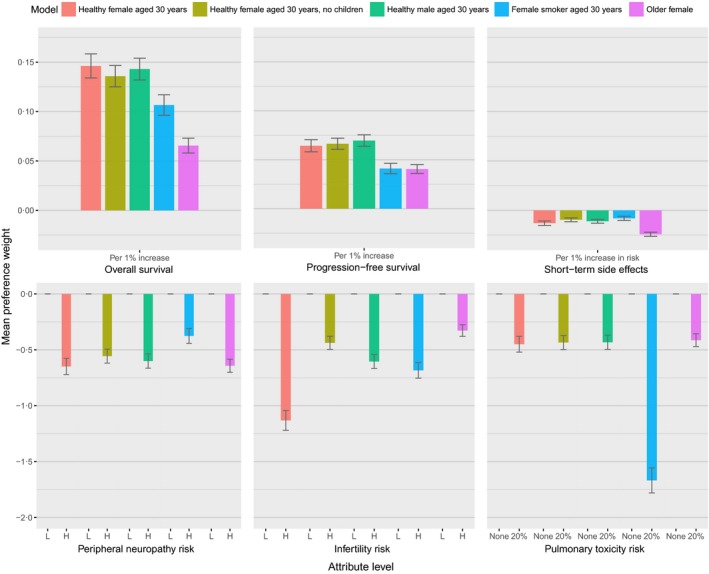
Mean preference weights for the physician discrete‐choice experiment.
Relative importance of attributes – preference weights
Among both patients and physicians, survival attributes were consistently ranked high in importance given the levels presented in the DCE (Figs 3 and 4). Patients attributed the highest preference weight to PFS (24%) followed by OS (19%). This preference for PFS over OS was observed both in the main analysis of all patients, as well as for sub‐analyses of patients in early or intermediate/advanced stage HL, male or female patients and patients aged under 55 years. Only patients aged 55 years or older put a higher weight on OS (33%) than on PFS (27%). The largest difference in preference weight between PFS and OS was observed in the sub‐analysis of female patients, where PFS was weighted at 25% and OS at only 13%. In this group, a reduction in risk of pulmonary toxicity was the second most heavily weighted preference (20%). The relative importance of infertility was 17·5% among patients under 55 years of age compared to 1·6% among patients 55 years and older. When comparing patients who had not yet started treatment with those who were on or had completed treatment at the time of the survey, the treatment‐naïve patients put overall less emphasis on side effects than the patients with experience of treatment. Among the side effects, infertility gained in importance among those with treatment experience. The importance of side effects, especially infertility and neuropathy, was even greater among patients who had not achieved remission or relapsed.
Figure 3.
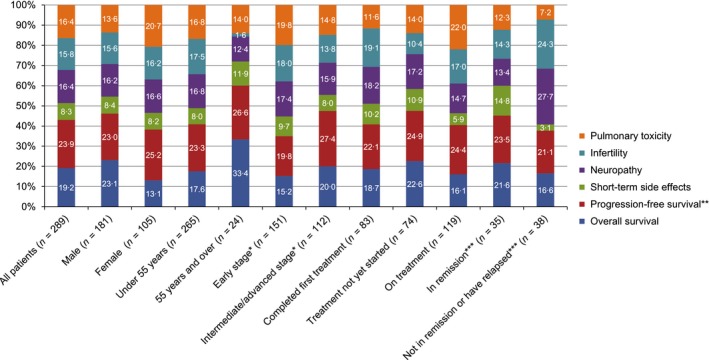
Relative importance (%) of the first‐line treatment for Hodgkin lymphoma attributes to treatment decision for patients, as determined by mixed logit model, overall and stratified by patient gender, age, disease stage, treatment and disease status.*Early stage = stage I, stage II and early stage; Intermediate/advanced stage = stage III, stage IV, intermediate and advanced; **Described as the risk of relapse or progression within 5 years for patients; ***These strata apply only to patients who had completed treatment.
Figure 4.
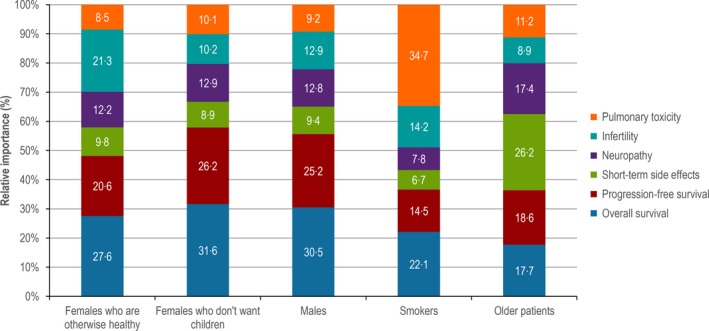
Relative importance (%) of the first‐line Hodgkin lymphoma treatment attributes to treatment decision for physicians (n = 281), as determined by the mixed logit model and presented according to the five patient profiles.
In contrast to the preference of PFS over OS observed among patients, among physicians, OS generally was attributed a higher preference weight than PFS. The only patient profiles where either PFS or OS was not the most important attribute was patients who were smokers, where pulmonary toxicity was considered most important, and patients older in age, for which the highest preference weight was for a reduction in risks of side effects requiring hospitalisation. For the older patient, physicians weighted PFS slightly higher than OS. Short‐term side effects were consistently attributed a lower importance in all other patient profiles. Similarly, pulmonary toxicity was ranked low for most profiles. In contrast, patients ranked this attribute the highest after survival‐related. For physicians, infertility was the second most important attribute after OS when considering a patient profile of a female where no information about desire for children was provided. However, differences were noted in physician preferences by country for this profile, with infertility having the highest relative importance for physicians from the UK, but having the third highest relative importance after OS and PFS for physicians from Germany and France (data not shown).
Relative importance of attributes – willingness to trade
The relative importance of attributes can also be expressed in terms of participants’ willingness to accept changes in one attribute as a trade‐off to a change in a different attribute. As OS and PFS (physicians)/risk of relapse/progression (patients) were the most important attributes to both patients and physicians, we looked at the percentage change in these attributes that participants required in order to accept an increase in the risk of an adverse event attribute (Tables 4 and 5).
Table 4.
Increase in overall survival or decrease in risk of relapse/progression required to accept an increase in risk of infertility, peripheral neuropathy, pulmonary toxicity or hospitalisation from the patient perspective
| Required % increase in overall survival from 84% to accept risk | Required % decrease in risk of relapse/progression from 26% to accept risk | |
|---|---|---|
| Patients would be willing to accept | ||
| High risk of infertility | 8·2 | 14·6 |
| High risk of peripheral neuropathy | 8·5 | 15·1 |
| 20% risk of pulmonary toxicity | 8·5 | 15·1 |
| 20% increase in risk of hospitalisation | 2·2 | 3·8 |
Table 5.
Increase in overall survival or decrease in risk of relapse/progression required to accept an increase in risk of infertility, peripheral neuropathy, pulmonary toxicity or hospitalisation from the physician perspective, by patient profile
| Required % increase in OS from 84% to accept risk | Required % increase in PFS from 68% to accept risk | |
|---|---|---|
| Physicians would be willing to accept | ||
| For otherwise healthy 30‐year‐old female patient | ||
| High risk of infertility | 7·7 | 17·6 |
| High risk of peripheral neuropathy | 4·4 | 10·1 |
| 20% risk of pulmonary toxicity | 3·1 | 7·0 |
| 20% increase in risk of hospitalisation | 1·8 | 4·0 |
| For otherwise healthy 30‐year‐old female patient who wants no children | ||
| High risk of infertility | 3·2 | 6·6 |
| High risk of peripheral neuropathy | 4·1 | 8·4 |
| 20% risk of pulmonary toxicity | 3·2 | 6·6 |
| 20% increase in risk of hospitalisation | 1·4 | 2·9 |
| For otherwise healthy 30‐year‐old male patient | ||
| High risk of infertility | 4·2 | 8·7 |
| High risk of peripheral neuropathy | 4·2 | 8·6 |
| 20% risk of pulmonary toxicity | 3·0 | 6·2 |
| 20% increase in risk of hospitalisation | 1·5 | 3·2 |
| For 30‐year‐old female patient who smokes | ||
| High risk of infertility | 6·4 | 16·7 |
| High risk of peripheral neuropathy | 3·5 | 9·1 |
| 20% risk of pulmonary toxicity | 15·6 | 40·6 |
| 20% increase in risk of hospitalisation | 1·5 | 3·9 |
| For 60‐year‐old female patient | ||
| High risk of infertility | 5·0 | 8·1 |
| High risk of peripheral neuropathy | 9·8 | 15·9 |
| 20% risk of pulmonary toxicity | 6·3 | 10·2 |
| 20% increase in risk of hospitalisation | 7·4 | 11·9 |
OS, overall survival; PFS, progression free survival.
Patients were willing to accept a treatment with higher risk of infertility (levels: high risk versus low risk), peripheral neuropathy (levels: high risk versus low risk) and pulmonary toxicity (levels: 20% risk versus no risk) if the treatment provided an increase in OS of 8·2–8·5% from 84%, or if the treatment provided a decrease in the risk of relapse/progression of 14·6–15·1% from 26% (Table 4). A lower increase in OS (2·2%) or decrease in risk of progression/relapse (3·8%) was required for patients to be willing to accept a 20% increase in the risk of hospitalisation due to adverse events.
Physicians’ differential approach to the presented patient types is also reflected in physicians’ willingness to accept higher levels of risk of infertility, peripheral neuropathy, pulmonary toxicity or hospitalisation due to adverse events in exchange for higher OS or PFS. While infertility was more easily accepted in a patient who does not want children or an older person, the risk of short‐term side effects was more accepted in the case of younger patients. An increase in pulmonary toxicity was generally more readily accepted than an increase in peripheral neuropathy, except in the case of a patient who smokes (Table 5).
Sensitivity analyses
Among the physicians, 21·3% chose the non‐dominant scenario for at least one patient profile, and 56·3% changed their response at least for one patient type between the two repeats of the same comparison. Among patients, a similar proportion (23·6%) chose the non‐dominant scenario, and 29·7% changed their choice between the two repeats of the same comparison. The same trends in preferences were observed in the sensitivity analyses as in the primary analysis. However, the magnitude of these preferences was diminished among the sensitivity 1 population (all participants included, rather than only the ones with logical responses) and enhanced among the sensitivity 2 population (participants with logical and consistent responses, rather than logical responses regardless of consistency) compared to the primary analysis population (data not shown).
Discussion
This study evaluated the importance of attributes of first‐line HL treatments that impact patient and physician treatment preferences. With an increasing emphasis on patient‐centric care in haemato‐oncology and other disciplines, an understanding of patient as well as physician treatment preferences, and the identification of discrepancies between attributes of treatments preferred by patients and physicians can contribute to improved care.
With the range of levels presented in the DCE, 5‐year PFS and OS were observed to be the most important attributes to patients when selecting treatment preferences. The magnitude of the coefficients from the MXL model revealed that, in the context of the present DCE, a 1% change in 5‐year OS was more important to patients than a 1% change in 5‐year PFS; however, the overall preference weight was higher for PFS than for OS. This is due to the fact that the weights are based on the coefficients per 1% increase multiplied by the maximum range between attribute levels (22% for PFS, but only 10% for OS).
Differences in patient preferences were observed according to patient subgroups. Male patients ranked PFS and OS as nearly equally most important, while female patients were much more likely to base a large part of their decision on PFS. The importance of infertility differed with age, being of very little importance to patients aged 55 years and older.
The results of the physician survey demonstrate the importance of patient characteristics to physicians’ treatment preferences, as the preference weights for each level and the relative importance of the treatment attributes were observed to vary according to the patient description. PFS and OS most strongly influenced physicians’ treatment choices when considering younger female patients who did not want children and males approximately 30 years old. The risk of infertility was more important to physicians’ treatment decision than PFS, but less important than OS, when considering younger women whose fertility preferences were unknown. This difference was driven by UK physicians, among whom risk of infertility most affected their treatment decision; whereas in Germany and France, PFS was more important than fertility. This may motivate or reflect the more common use of ABVD rather than BEACOPPescalated in the UK compared to France and Germany. In all countries, the risk of pulmonary toxicity most strongly influenced treatment decisions when physicians were considering patients who were smokers. Short‐term side effects played the largest role in the treatment decision when physicians were considering the treatment of older patients.
Patient and physician preferences generally aligned, with survival outcomes having a strong influence on preferences for both; however, physicians tended to rank OS over PFS, while the opposite was true for patients. Two additional notable differences were observed. First, physicians generally attributed less importance to long‐term pulmonary toxicity than patients. Second, physicians considered side effects to be the most important attribute when considering older patients, whereas side effects had the lowest relative importance of all attributes among patients 55 years of age and older with less than 10% of their treatment choice based on this attribute.
While numerous preference studies have been conducted in oncology, there is a paucity of preference data specific to the treatment of malignant lymphomas. Shafey et al (2011) assessed patient and physician preferences for treatments for relapsed follicular lymphoma, including standard chemotherapy, radioimmunotherapy, high dose chemotherapy and autologous stem cell transplant. This study in indolent lymphoma found that treatment choice was largely influenced by survival free of relapse, with both patients and physicians requiring larger increases in PFS in order to accept more toxic treatment options (Shafey et al, 2011). These results align with the findings in the current study in more aggressive but highly curable HL, where efficacy outcomes were of most importance to patients and also to physicians, except when considering older patients or patients with relevant comorbidities. In particular, the results of our DCE showed that patients put great importance on PFS (phrased for the patients as “likelihood of your HL coming back or getting worse”), which had a higher preference weight than OS especially among women. A non‐DCE survey among HL survivors who had participated in interventional trials of HL treatment also showed a preference for PFS over OS, with participants expressing more concern about relapsing than dying from HL (Buerkle et al, 2016). That survey also showed a willingness to accept side effects for an efficacious treatment; only 2% of participants reported that they would have chosen a slightly less effective therapy to avoid side effects.
While not specific to lymphoma, Fried et al (2002) assessed treatment preferences among seriously ill patients, including those with advanced‐stage cancer, and found that treatment preferences were largely influenced by the likelihood of outcomes occurring. The probability of an adverse outcome influenced the number of patients selecting treatment, unless the probability was ≤10%. In line with the present study, Fried et al (2002) found that patients were generally willing to accept treatments with high burden and high risk of adverse outcomes to obtain better efficacy outcomes.
We consider that an important part of this first DCE study in HL was the inclusion of multiple sources of information to inform the DCE attributes and level selection, including a literature review, clinical expert interviews and patient qualitative interviews. The DCE language was reviewed among physicians and patients in these interviews and the survey was pilot tested to ensure the attributes, patient profiles, treatment scenarios, and responses options were logical and comprehensive to participants. The physician survey included five different patient profiles, differing in one patient characteristic at a time, to evaluate the impact of individual patient characteristics on physician preferences. The patient characteristics included in the profiles were selected based on literature review and clinical expert feedback. Lastly, logic and consistency tests were built into the DCE. Sensitivity analyses showed that the overall trends in preferences were consistent regardless of whether participants who failed the logic test and/or showed inconsistent answers were excluded from the analysis.
Whilst the study objective is specific to preferences for advanced‐stage HL treatment, the majority of patients recruited were diagnosed in the early stages of the disease in order to achieve the target sample size. For analysis purposes, the heterogeneous groups of self‐reported intermediate‐ and advanced‐stage disease patients were pooled because both groups represent patients in need of innovative effective first‐line therapies. Additionally, the patient sample also included patients who had previously received treatment in order to obtain the target sample size. Therefore, the preferences of patients surveyed may not be generalizable to patients with advanced‐stage HL who are making a decision about first‐line HL treatments, but represent a more general perspective of preferences with regards to attributes of common first‐line treatments for HL. Due to the rather young patient population in the present study as well as limited resilience of the self‐reported information on disease extent, first‐line therapy and current disease status, generalizability of results is additionally hampered (Cancer Research UK, 2017).
For the purpose of comprehension, the PFS attribute description was altered slightly for the patient survey. This alteration may lead to the rating and relative importance of the attributes not being directly comparable between the patients and physicians. The attribute levels were generated based on available data that was comparable between treatments of interests. The incidence of specific toxicities, such as infertility with ABVD or its variants might be misrepresented due to insufficient data within the levels for therapeutic intensity. In contrast to current standard of care, the treatments of interest did not include PET‐driven individualized approaches. Lastly, the DCE exercise presents hypothetical scenarios to patients and physicians. Real world treatment preferences and actual treatment choices in particular, may differ depending on factors not described in the DCE scenarios. Based on clinical expert feedback, patients in the real‐world settings in Germany, France and the UK may not be offered a real choice between first‐line HL treatments, and physicians may not be able to select their preferred treatment based on hospital and country practice guidelines as well as reimbursement plans they are required to follow.
In summary, we found that patients preferred first‐line HL treatments that offered lower risk of HL returning and longer survival and were willing to accept increased side effects if they were associated with an increase in chances of survival. Patients generally put more weight on an increase in PFS than OS. Physicians based their preferences for specific treatment attributes on the patient profile presented, but overall valued OS over PFS.
Disclosures
This study was funded by Millennium Pharmaceuticals, Inc. EZ, DH, AG and MD were employed and had equity ownership with Millennium Pharmaceutical. PJB, AS and TI provided consulting services to and received honoraria from Millennium Pharmaceutical. SM, JBW, KM and SG were employees of ICON plc, a Contract Research Organisation contracted by Millennium Pharmaceutical to conduct this study.
Acknowledgements
PJB, TI, AS, MD, EZ, AG, DH, SM, JBW, KM and SG designed the research study. MD, SM, JBW, KM and SG performed the research. SM, JBW and SG analysed the data. PJB, TI, AS, MD, SM, and KM wrote the paper. EZ, AG, DH JBW and SG reviewed and revised the manuscript. All authors have approved the manuscript. The authors thank Mary He (ICON plc.) for creating the colour figures for this manuscript.
References
- Ansell, S. , Younes, A. , Conners, J. , Gallamini, A. , Kim, W. , Friedberg, J. , Feldman, T. , Collins, G. , Bartlett, N. , Wang, J. , Brady, K. , Sachs, J. , Huebner, D. , Hunder, N. & Radford, J. (2014) Phase 3 study of brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) versus doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as front‐line treatment for advanced classical Hodgkin lymphoma (HL): Echelon‐1 study. Journal of Clinical Oncology, 32 (15_suppl), TPS8613. [Google Scholar]
- Behringer, K. , Mueller, H. , Goergen, H. , Thielen, I. , Eibl, A.D. , Stumpf, V. , Wessels, C. , Wiehlputz, M. , Rosenbrock, J. , Halbsguth, T. , Reiners, K.S. , Schober, T. , Renno, J.H. , von Wolff, M. , van der Ven, K. , Kuehr, M. , Fuchs, M. , Diehl, V. , Engert, A. & Borchmann, P. (2013) Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. Journal of Clinical Oncology, 31, 231–239. [DOI] [PubMed] [Google Scholar]
- Bhakta, N. , Liu, Q. , Yeo, F. , Baassiri, M. , Ehrhardt, M.J. , Srivastava, D.K. , Metzger, M.L. , Krasin, M.J. , Ness, K.K. , Hudson, M.M. , Yasui, Y. & Robison, L.L. (2016) Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. The Lancet Oncology, 17, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchmann, P. , Goergen, H. , Kobe, C. , Lohri, A. , Greil, R. , Eichenauer, D.A. , Zijlstra, J.M. , Markova, J. , Meissner, J. , Feuring‐Buske, M. , Huttmann, A. , Dierlamm, J. , Soekler, M. , Beck, H.J. , Willenbacher, W. , Ludwig, W.D. , Pabst, T. , Topp, M.S. , Hitz, F. , Bentz, M. , Keller, U.B. , Kuhnhardt, D. , Ostermann, H. , Schmitz, N. , Hertenstein, B. , Aulitzky, W. , Maschmeyer, G. , Vieler, T. , Eich, H. , Baues, C. , Stein, H. , Fuchs, M. , Kuhnert, G. , Diehl, V. , Dietlein, M. & Engert, A. (2018) PET‐guided treatment in patients with advanced‐stage Hodgkin's lymphoma (HD18): final results of an open‐label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet, 390, 2790–2802. [DOI] [PubMed] [Google Scholar]
- Buerkle, C. , Goergen, H. , Muller, H. , Brockelmann, P. , Kreissl, S. , Fuchs, M. , Engert, A. , Borchmann, P. & Behringer, K. (2016) Patient preferences – survey of Hodgkin Lymphoma survivors on treatment‐associated burden and side effects. Haematologica, 101 (suppl 5), 61, T025. [Google Scholar]
- Cancer Research UK . (2017). Hodgkin lymphoma incidence statistics [Online]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/hodgkin-lymphoma/incidence#heading-One.
- Carde, P. , Karrasch, M. , Fortpied, C. , Brice, P. , Khaled, H. , Casasnovas, O. , Caillot, D. , Gaillard, I. , Bologna, S. , Ferme, C. , Lugtenburg, P.J. , Morschhauser, F. , Aurer, I. , Coiffier, B. , Meyer, R. , Seftel, M. , Wolf, M. , Glimelius, B. , Sureda, A. & Mounier, N. (2016) Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, International Prognostic Score >/= 3, high‐risk Hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. Journal of Clinical Oncology, 34, 2028–2036. [DOI] [PubMed] [Google Scholar]
- Connors, J. , Jurczak, W. , Straus, D. , Ansell, S. , Kim, W.S. , Gallamini, A. , Younes, A. , Alekseev, S. , Illes, A. , Picardi, M. , Lech‐Maranda, E. , Okie, Y. , Feldman, T.A. , Smolewski, P. , Savage, K. , Bartlett, N. , Walewski, J. , Chen, R. , Ramchandren, R. , Zinzani, P.L. , Cunningham, D. , Heo, D.S. , Rosta, A. , Josephson, N. , Ruffner, K. , Sachs, J. , Liu, R. , Jolin, H. , Huebner, D. & Radford, J. (2017) Brentuximab vedotin plus doxorubicin, vinblastine, dacarbazine (A+AVD) As frontline therapy demonstrates superior modified progression‐free survival versus ABVD in patients with previously untreated stage III or IV Hodgkin lymphoma (HL): the phase 3 Echelon‐1 study. Blood, 130, 6.28684448 [Google Scholar]
- Eichenauer, D.A. , Plütschow, A. , Kreissl, S. , Sökler, M. , Hellmuth, J.C. , Meissner, J. , Mathas, S. , Topp, M.S. , Behringer, K. , Klapper, W. , Kuhnert, G. , Dietlein, M. , Kobe, C. , Fuchs, M. , Diehl, V. , Engert, A. & Borchmann, P. (2017) Incorporation of brentuximab vedotin into first‐line treatment of advanced classical Hodgkin's lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. The Lancet Oncology, 18, 1680–1687. [DOI] [PubMed] [Google Scholar]
- Eichenauer, D.A. , Aleman, B.M.P. , Andre, M. , Federico, M. , Hutchings, M. , Illidge, T. , Engert, A. & Ladetto, M. (2018) Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology. 10.1093/annonc/mdy080 [DOI] [PubMed] [Google Scholar]
- EMA . (2013). The patient's voice in the evaluation of medicines [Online]. European Medicines Agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/10/WC500153276.pdf.
- FDA . (2017). Patient preference initiative [Online]. Food and Drug Administration. Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHPatientEngagement/ucm462830.htm.
- Federico, M. , Luminari, S. , Iannitto, E. , Polimeno, G. , Marcheselli, L. , Montanini, A. , La, S.A. , Merli, F. , Stelitano, C. , Pozzi, S. , Scalone, R. , Di, R.N. , Musto, P. , Baldini, L. , Cervetti, G. , Angrilli, F. , Mazza, P. , Brugiatelli, M. & Gobbi, P.G. (2009) ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. Journal of Clinical Oncology, 27, 805–811. [DOI] [PubMed] [Google Scholar]
- Fried, T.R. , Bradley, E.H. , Towle, V.R. & Allore, H. (2002) Understanding the treatment preferences of seriously ill patients. New England Journal of Medicine, 346, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Johnson, P. , Federico, M. , Kirkwood, A. , Fossa, A. , Berkahn, L. , Carella, A. , D'Amore, F. , Enblad, G. , Franceschetto, A. , Fulham, M. , Luminari, S. , O'Doherty, M. , Patrick, P. , Roberts, T. , Sidra, G. , Stevens, L. , Smith, P. , Trotman, J. , Viney, Z. , Radford, J. & Barrington, S. (2016) Adapted treatment guided by interim PET‐CT scan in advanced Hodgkin's lymphoma. New England Journal of Medicine, 374, 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreissl, S. , Mueller, H. , Goergen, H. , Mayer, A. , Brillant, C. , Behringer, K. , Halbsguth, T.V. , Hitz, F. , Soekler, M. , Shonukan, O. , Rueffer, J.U. , Flechtner, H.H. , Fuchs, M. , Diehl, V. , Engert, A. & Borchmann, P. (2016) Cancer‐related fatigue in patients with and survivors of Hodgkin's lymphoma: a longitudinal study of the German Hodgkin Study Group. The Lancet Oncology, 17, 1453–1462. [DOI] [PubMed] [Google Scholar]
- Mounier, N. , Brice, P. , Bologna, S. , Briere, J. , Gaillard, I. , Heczko, M. , Gabarre, J. , Casasnovas, O. , Jaubert, J. , Colin, P. , Delmer, A. , Devidas, A. , Bachy, E. , Nicolas‐Virelizier, E. , Aoudjhane, A. , Humbrecht, C. , Andre, M. & Carde, P. (2014) ABVD (8 cycles) versus BEACOPP (4 escalated cycles >/= 4 baseline): final results in stage III‐IV low‐risk Hodgkin lymphoma (IPS 0‐2) of the LYSA H34 randomized trial. Annals of Oncology, 25, 1622–1628. [DOI] [PubMed] [Google Scholar]
- Schaapveld, M. , Aleman, B.M. , van Eggermond, A.M. , Janus, C.P. , Krol, A.D. , van der Maazen, R.W. , Roesink, J. , Raemaekers, J.M. , de Boer, J.P. , Zijlstra, J.M. , van Imhoff, G.W. , Petersen, E.J. , Poortmans, P.M. , Beijert, M. , Lybeert, M.L. , Mulder, I. , Visser, O. , Louwman, M.W. , Krul, I.M. , Lugtenburg, P.J. & van Leeuwen, F.E. (2015) Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. New England Journal of Medicine, 373, 2499–2511. [DOI] [PubMed] [Google Scholar]
- SEER . (2016).SEER cancer statistics factsheets: Hodgkin lymphoma [Online]. Bethesda, MD: National Cancer Institute; Available from: https://seer.cancer.gov/statfacts/html/hodg.html [Accessed 27 April 2014]. [Google Scholar]
- Shafey, M. , Lupichuk, S.M. , Do, T. , Owen, C. & Stewart, D.A. (2011) Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplantation, 46, 962–969. [DOI] [PubMed] [Google Scholar]
- Skoetz, N. , Trelle, S. , Rancea, M. , Haverkamp, H. , Diehl, V. , Engert, A. & Borchmann, P. (2013) Effect of initial treatment strategy on survival of patients with advanced‐stage Hodgkin's lymphoma: a systematic review and network meta‐analysis. The Lancet Oncology, 14, 943–952. [DOI] [PubMed] [Google Scholar]
- Younes, A. , Connors, J.M. , Park, S.I. , Fanale, M. , O'Meara, M.M. , Hunder, N.N. , Huebner, D. & Ansell, S.M. (2013) Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open‐label, dose‐escalation study. The Lancet Oncology, 14, 1348–1356. [DOI] [PubMed] [Google Scholar]


