Figure 2.
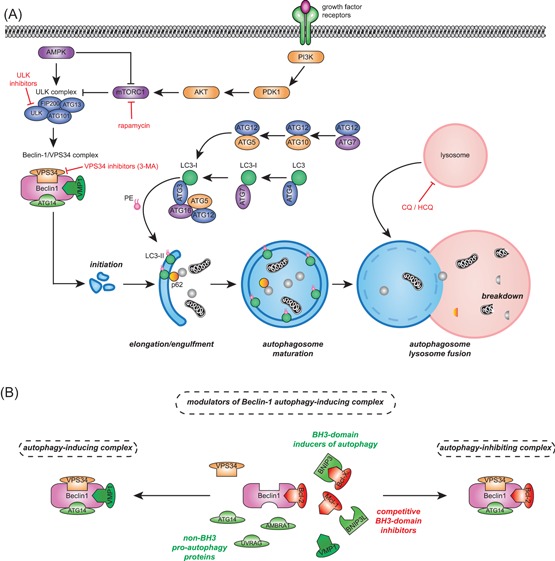
The autophagy pathway. A, The activation of autophagy is initiated by the reduced activity of the mechanistic target of rapamycin complex 1 (mTORC1) complex due to activated adenosine monophosphate‐activated protein kinase (AMPK) or decreased upstream growth signaling. mTORC1 is an inhibitor of the ULK complex, therefore reduced mTORC1 activity increases the activity of the ULK complex. The ULK complex together with the Beclin‐1/ VPS34 complex initiates the formation of autophagosomes. Dependent on the complex composition, Beclin‐1 can act as a molecular switch between autophagy and apoptosis (see B). The expansion and maturation of the autophagosomes is dependent on two ubiquitin‐like conjugation systems, which requires multiple autophagy proteins. First, ATG12‐ATG5 conjugate binds to ATG16, which stimulates LC3 lipidation. Second, LC3 is covalently conjugated to phosphatidylethanolamine (PE) generating LC3‐II, which is incorporated in the autophagosomal membrane. Incorporated LC3‐II is required for binding and internalization of adaptor proteins such as p62. Finally, the mature autophagosome fuses with lysosomes, after which its content is broken down by digestive enzymes. Indicated in red are pharmacological agents, chloroquine (CQ), hydroxychloroquine (HCQ), 3‐methyladenine (3‐MA), and ULK inhibitors, that inhibit autophagy. In addition, rapamycin activates autophagy by inhibiting mTORC1. B, Beclin‐1 is a core member of the VPS34/Beclin‐1 complex, which acts as a molecular switch in controlling autophagy downstream of the ULK1 complex. Depicted in red are the antiapoptotic members of the Bcl‐2 family BCL‐2, BCL‐XL, and MCL‐1 which can bind to Beclin‐1, through interaction with its BH3 domain, thereby inhibiting autophagy. Alternatively, Bcl‐2 interacting protein 3 (BNIP3) and Bcl‐2 interacting protein 3 like (BNIP3L; depicted in green) can competitively bind to antiapoptotic BLC‐2 members. Dissociation of antiapoptotic Bcl‐2 members from Beclin‐1, consequently activates autophagy. Other non‐BH3 proteins, also depicted in green, such as vacuole membrane protein 1 (VMP1), ATG14, UV radiation resistance‐associated gene (UVRAG), and activating molecule in Beclin‐1‐regulated autophagy protein 1 (AMBRA1) can also bind Beclin‐1, thereby activating autophagy. PDK1, pyruvate dehydrogenase kinase 1; PI3K, phosphoinositide 3‐kinase [Color figure can be viewed at wileyonlinelibrary.com]
