Figure 2.
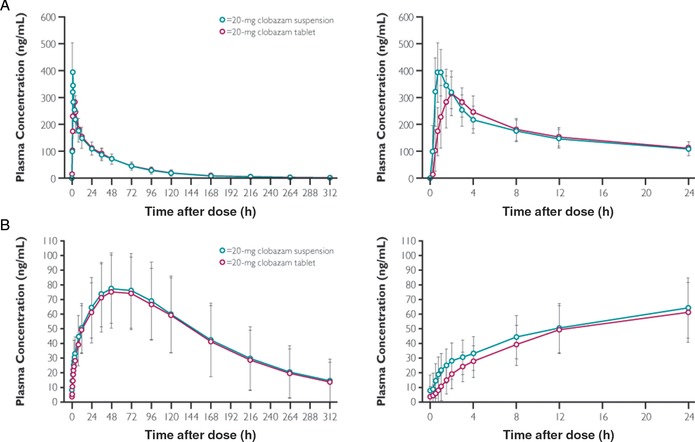
Mean (± SD) clobazam (A) and N‐desmethylclobazam (B) plasma concentrations following a single oral dose of 20‐mg clobazam suspension (test) or tablet (reference).32 In this randomized, open‐label, 2‐way crossover study, participants received a single dose of 20‐mg CLB oral suspension or tablet on study day 1 and day 15, with an intervening 14‐day washout period. PK parameters were measured from blood samples drawn predose (0 hours) and regularly through 312 hours after dose, quantified via LC‐MS/MS. The panels on the left show the entire time course; the panels on the right show finer detail for the plasma concentrations versus time in the first 24 hours. CLB, clobazam; LC, liquid chromatography; MS, mass spectrometry.
