Figure 2.
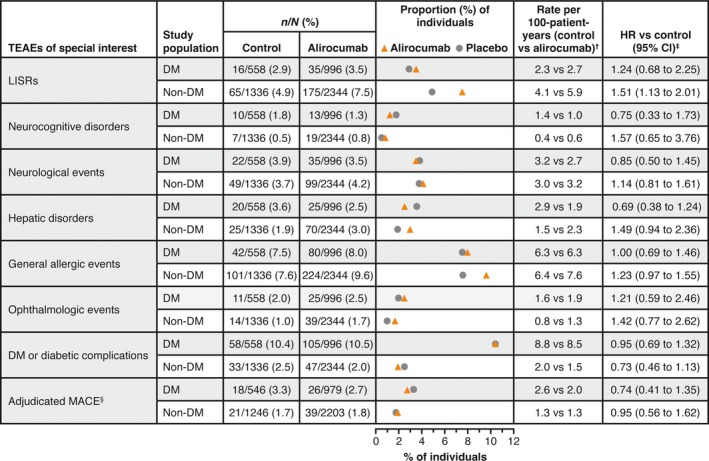
Adverse events of special interest according to diabetes status at baseline in the pool of 14 phase 2/3 trials (safety population). †Calculated as number of people with an event divided by total person‐years. For people with an event, the number of person‐years is calculated up to the date of the first event; for those without an event, it corresponds to the length of the treatment‐emergent adverse event (TEAE) period. ‡Calculated using a Cox model stratified on the study. §Major adverse cardiac events (MACE) were defined as coronary heart disease death, non‐fatal myocardial infarction, ischaemic stroke or unstable angina requiring hospitalization, and were adjudicated by a central Clinical Events Committee only in phase 3 trials. Local injection site reactions (LISRs) were selected using an electronic case report form‐specific tick box on the adverse event page in phase 3 studies and phase 2 study DFI12361, selected using Medical Dictionary of Regulatory Activities (MedDRA) high‐level term ‘injection site reaction’ in the other phase 2 studies. Neurocognitive disorder: events selected using a custom MedDRA query, based on the five following high‐level group terms: deliria (including confusion); cognitive and attention disorders and disturbances; dementia and amnestic conditions; disturbances in thinking and perception; and mental impairment disorders. Neurological events: standardized MedDRA queries ‘demyelination’ (broad and narrow), ‘peripheral neuropathy’ (broad and narrow), and ‘Guillain–Barre syndrome’ (broad and narrow), excluding the following preferred terms: ‘acute respiratory distress syndrome’, ‘asthenia’, ‘respiratory arrest’ and ‘respiratory failure’. Hepatic disorder: standardized MedDRA query ‘hepatic disorder’. General allergic events: selected using a custom MedDRA query with standardized MedDRA query ‘hypersensitivity’ (broad and narrow), excluding the following preferred terms linked to LISRs: (‘infusion site dermatitis’, ‘infusion site hypersensitivity’, ‘infusion site rash’, ‘infusion site urticaria,’ ‘injection site dermatitis’, ‘injection site hypersensitivity’, ‘injection site rash’, ‘injection site urticaria’ and ‘vasculitis’. Ophthalmologic events: standardized MedDRA queries ‘optic nerve disorders’ (broad and narrow), ‘retinal disorders’ (narrow) and ‘corneal disorders’ (narrow). Diabetes or diabetic complications: high‐level group term ‘diabetes complications’, high‐level term ‘diabetes mellitus’ and high‐level term ‘carbohydrate tolerance analyses (including diabetes)’, excluding preferred term ‘blood glucose decreased’. In study participants with diabetes at baseline, terms such as ‘diabetes mellitus’ indicate a worsening of the condition or loss of glycaemic control. DM, diabetes mellitus; HR, hazard ratio; TEAE, treatment‐emergent adverse event.
