Abstract
Aim
A standard approach to measure subcutaneous adipose tissue (SAT) using ultrasound has proved successful in adults, but has not been studied in children. This study addressed that gap in children aged three to five years.
Methods
In autumn 2016, 24 preschools in Southwest Germany, recruited via mail, agreed to take part in this study and 274 children (51.4% boys) with a mean age of 4.6 ± 0.7 years participated in measurements of SAT and anthropometry. Differences in measurements were explored between the sexes and anthropometric predictors of mean SAT thickness were identified. Intra‐observer reliability for ultrasound measurements of SAT was also assessed.
Results
The mean SAT thickness showed significant differences between the boys and girls (5.3 ± 2.0 and 6.3 ± 2.0 mm, respectively, p < 0.01). The children's body mass, height and sex explained 66% of the variance in the mean SAT thickness, as SAT was larger with a higher body mass, a smaller stature and in girls. Intra‐observer reliability resulted in an intra‐class correlation coefficient of 0.994 (p < 0.01) with a 95% confidence interval of 0.983–0.998.
Conclusion
Subcutaneous adipose tissue thickness differed between boys and girls with a mean age of 4.6 years. Intra‐observer reliability was excellent. This standardised approach enabled high‐precision measurements of SAT in a paediatric population.
Keywords: Anthropometric predictors, Body composition, Body fat, Subcutaneous adipose tissue, Ultrasound
Abbreviations
- BF%
Total body fat percentage
- BMI
Body mass index
- d
Mean subcutaneous adipose tissue thickness (of all eight sites)
- D
Sum of subcutaneous adipose tissue thickness of eight sites
- Excl
Subcutaneous adipose tissue excluding embedded fibrous structures
- Incl
Subcutaneous adipose tissue including embedded fibrous structures
- SAT
Subcutaneous adipose tissue
- WC
Waist circumference
Key notes.
This study measured subcutaneous adipose tissue (SAT) thickness using ultrasound in 274 preschool children, aged three to five years, from 24 preschools in Southwest Germany.
SAT thickness showed significant differences between boys and girls: it was larger with a higher body mass, a smaller stature and in girls.
Ultrasound provided accurate measurements of SAT with excellent intra‐observer reliability.
Introduction
Observational studies that examine the status of children's growth and health to identify the prevalence of overweight and obesity 1, 2 use different methods to determine excessive body weight and body fat. The same applies to health intervention studies that aim to prevent an unhealthy weight status 3, 4, For example, field methods that assess body composition comprise anthropometric characteristics which include height, body mass, waist circumference (WC) and indirect measurements of body fat, such as skinfold thickness 5, 6, 7, 8. Two types of measurement errors can arise when assessing body composition: methodological errors in collecting raw data and errors in the assumptions that are made when converting raw data into final values. The degree of error varies with the measurement method 7. Multi‐component models, especially the four‐component model, are considered to be most accurate and are used as reference methods against which other methods, especially field methods, are validated and compared 6, 7, 9. Multi‐component models combine measurements of several fat‐free body components, which require a large methodological effort. These include the need for multiple laboratory methods, additional time and added costs, which makes them impractical for large‐scale field studies that examine body composition in children 6, 7, 9.
A common and easy‐to‐use method to describe weight status and obesity is the body mass index (BMI), converted to sex‐ and age‐specific percentiles for children 10, 11. However, BMI does not consider body fat mass or its distribution 12, 13, 14. Further examining the body shape and distribution of body fat, WC is used to assess central adiposity in young children 7, 15. As with BMI, percentiles are used to determine children with increased risk, but there are no universally accepted cut‐off values 16. WC is a height‐dependent variable and the exact measurement location of the WC can vary 15, 16, 17. Considering both height and WC, the waist‐to‐height ratio, measured as WC in cm/height in cm, identifies central adiposity independent of age 15, 17. However, its use is not recommended for children under the age of six 15 and it is not superior to BMI or WC in predicting the total body fat percentage (BF%) in children aged three to seven 18. Another approach to assessing body fat at various body sites are skinfold thickness measurements. These measurements allow an evaluation of regional subcutaneous fat and are often used to estimate BF% 6, 7. The sum of skinfolds has been shown to outperform BMI and WC in the prediction of BF% in children aged six to 13 19. This method is convenient as well as cost‐efficient and therefore attractive for large studies 6, 7, 19. However, methodological errors in collecting raw data can arise due to site‐specific compression of adipose tissue and individual variations in elastic properties of the skin 6, 20. Applying population‐specific equations to estimate BF% from skinfold measurements on an individual level can add to further error in the assumptions made and is not recommended 7, 21.
Studies have shown the advantages of using ultrasound to measure subcutaneous adipose tissue (SAT) as part of a body composition assessment 22, 23, 24. Ultrasound enables the measurement of uncompressed SAT thickness and gives more accurate information on regional fat distribution than skinfolds 20. With the ready availability of small and portable devices, ultrasound measurements of SAT can easily be used in the field 23, 24, 25.
A standardised approach for performing accurate and reliable measurements of SAT has been developed by the International Olympic Committee Working Group on Body Composition, Health and Performance 23. This approach includes site marking, imaging and image evaluation. It enables cross‐sectional and longitudinal studies of fat patterning in lean and obese individuals, as well as in athletes 23, 24, 25. The underlying principle of brightness‐mode ultrasound imaging is the reflection of ultrasound beams from tissue borders in the path of the transmitted beam. Ultrasound pulses passing through body tissues are reflected, depending on the difference in the acoustic impedance between tissue interfaces, resulting in low‐intensity or high‐intensity echoes 26. The intensity of the echoes is represented in the brightness of the image on the screen. Tissue interfaces with low ultrasound reflexion appear dark on the screen and high‐intensity echoes appear bright 20, 26. Figure 1 shows the difference in reflected brightness depending on the tissues and their interfaces, namely skin, fat and muscle. The distance between the dermis and adipose tissue interface and the adipose tissue and muscle fascia interface depicts the measured adipose tissue using ultrasound.
Figure 1.
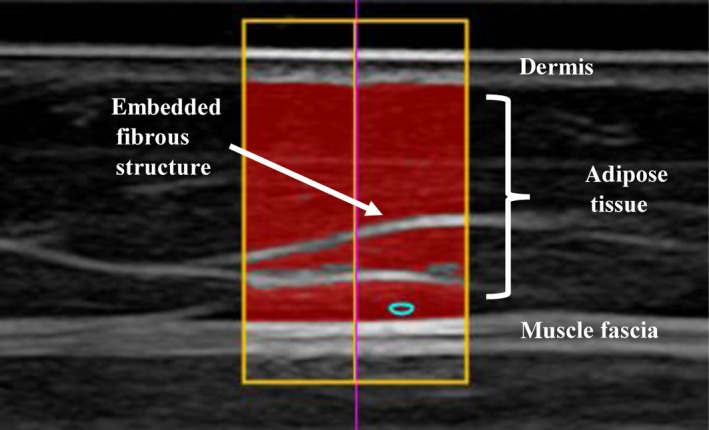
Example of an ultrasound image at the front thigh with structures of relevance marked for image evaluation. In the highlighted area, the evaluation algorithm automatically measured a mean subcutaneous adipose tissue thickness of 8.18 mm including embedded structures (7.19 mm without embedded structures) along 123 vertical lines from the lower border of the dermis to the upper border of the muscle fascia.
Using the speed of sound in fat (1450 m/s) to calculate the tissue layer thickness, the accuracy of fat measurements depends primarily on the ultrasound image resolution 23, 26. The image resolution ranges from 0.1 mm using an 18 MHz linear transducer to 0.3 mm at 6 MHz 23, 24. Müller et al. discussed the accuracy limitations in detail and stated that measurement accuracy was not determined by technical limitations of ultrasound imaging, but by biological factors, such as furrowed borders of SAT. Their arguments hold true for all biological tissues independent of a subject's age 23.
Due to the above‐mentioned shortcomings of commonly used methods to assess body fat in children, and the advantages of ultrasound, the primary aim of this study was to apply this approach as part of a larger field study in preschool children, aged three to five years. Potential sex differences in anthropometry and SAT were explored and predictors for mean SAT thickness were identified. The secondary objective was to evaluate intra‐observer reliability in the ultrasound measurements using the standardised procedure in a paediatric population 23.
Methods
Experimental design and study population
In this study, baseline data from a sub‐sample of the health survey that evaluated the preschool‐based intervention programme Join the Healthy Boat in Southwest Germany were used. A detailed description of the programme as well as the study design and protocol has previously been published 4. The study was registered at the German Institute of Medical Documentation and Information, Cologne, Germany (ID: DRKS00010089) and approved by the ethics committee of Ulm University (application number 188/15). All preschools in Baden‐Wuerttemberg that had not participated in the programme by the spring of 2016 received written information about the study via mail and were given the opportunity to register. From these preschools, 62 registered to participate, and written, informed consent provided by parents resulted in a total of 1021 children participating in the study. Parents were informed about each measurement by means of a survey brochure and were given contact information for the study's coordination office in case they had any questions. In addition to the general health survey, the parents had the opportunity to allow their children to take part in various sub‐studies and one of those was this analysis of SAT using ultrasound. The parents of 710 children agreed that their child could take part in the ultrasound examination. For logistical reasons 24 preschools, with 383 children eligible for examination, were selected and 274 children (51.4% boys) were examined. Children participating in the ultrasound measurements were older than those who did not: 4.6 years ± 0.7 versus 4.5 years ± 0.8 (p = 0.026). Except for one boy, who was of African ancestry, all children were healthy and Caucasian. The discrepancy in numbers can be attributed to children not being present or willing to participate on the day ultrasound measurements were taken (n = 54) or all other anthropometric measurements were performed (n = 42). Measurements could not be taken from the remaining 13 children for a variety of reasons, such as a change in the child's status at preschool, inappropriate attire or an inability to measure because of a plaster cast. Anthropometric and ultrasound measurements were conducted on consecutive days and on the preschools’ premises. All measurements were performed by the same personnel throughout the study.
Anthropometric assessment
Height was measured using a Seca 217 portable stadiometer (Seca, Hamburg, Germany) to the nearest 0.1 cm. Body mass was measured barefoot and in underwear using a Seca 862 electronic scale to the nearest 0.05 kg (Seca, Hamburg, Germany). BMI was calculated according to the equation kg/m2 and converted to age‐specific and sex‐specific BMI percentiles using German reference values 11. WC was measured twice to the nearest 0.1 cm at the mid‐point between the lower costal border, 10th rib and the iliac crest, using a Lufkin W606P flexible steel tape (Apex Tool Group, Sparks Glencoe, MD, USA). Mean values were used for further analysis.
Ultrasound measurements
Ultrasound measurements of SAT were performed in accordance with the International Association of Sciences in Medicine and Sports and the protocol published in 2016 23. The examinations were conducted by a certified investigator from the above‐named association. Using a transportable Philips CX50 ultrasound system, with a 10/15 MHz linear transducer L12‐3 transducer (Philips Ultrasound, Bothell, WA, USA), measurements were performed with the participant lying in a supine, prone or rotated position with the transducer held longitudinally in the direction of the underlying muscle. Site marking was performed on the right side of the body and in reference to the participant's height (cm). The following sites were marked: upper abdomen, lower abdomen, erector spinae, distal triceps, brachioradialis, lateral thigh, front thigh and medial calf. In a few cases, where the marked site was too close to a nearby joint, the site was moved one centimetre distally. This was necessary at the brachioradialis and medial calf sites to ensure a clear image of SAT with parallel structures of the skin and underlying SAT and muscle fascia (Fig. 1). A thick layer of gel (3–5 mm) was applied onto the transducer to ensure uncompressed measurement of SAT, which was verified in each measurement by means of a black band above the skin that could be seen on the ultrasound screen. After each examination day, the captured images were imported into the FAT 3.1 image evaluation software (Rotosport, Stattegg, Austria) and evaluated in a blind‐rating situation. The images contained no information on the subject. The software includes a semi‐automatic distance evaluation algorithm for multiple thickness measurements in a given ultrasound image, where the speed of sound is set to that of adipose tissue, namely 1450 m/s. The software reports thickness values for each individual site, the sum of all eight sites (D) as well as the mean thickness of these sites (d). It also enables a distinction in SAT values, both including embedded fibrous structures (D Incl, d Incl) and excluding embedded fibrous structures (D Excl, d Excl). A detailed description and directions for site marking and image capturing have previously been published 23.
Reliability study
Intra‐observer reliability measurements were performed on 16 children (56.3% boys), at one of the participating preschools, to compare the sums of SAT D Incl measured twice on two consecutive days. Site marking, imaging and image evaluation were performed on both days in accordance with the above‐mentioned procedure.
Statistical analysis
All data analyses were performed using SPSS 21 (IBM Corp, Armonk, NY, USA). Descriptive statistics – mean values and standard deviations – were calculated for the total sample and for boys and girls separately. The Kolmogorov–Smirnov test was used to identify a normal distribution of variables. Mean values of anthropometric characteristics as well as the ultrasound variables between boys and girls were subsequently compared with a t‐test for independent samples and the Mann–Whitney U‐test, respectively. Pearson's product‐moment correlation coefficients determined whether there were relationships between anthropometric characteristics and mean SAT (d Incl). A hierarchical linear regression analysis using forced entry was performed to evaluate which of the anthropometric traits could predict mean SAT thickness (d Incl). Body mass, height, WC and sex were used as predictor variables in the analysis. Multi‐collinearity among the predictor variables was tested, which is why WC was removed in the final analysis. Adding WC to the model caused the variance inflation factor of the variable body mass to increase to 10.3 and the mean value to 5.1. In the final analysis to predict d Incl, body mass was entered first, followed by height and sex as the last predictor variable. The level of significance was set at p ≤ 0.05.
For the intra‐observer reliability analysis, the intra‐class correlation coefficient and its 95% confidence interval (95% CI) were calculated based on a two‐way mixed effects model, single rating and absolute agreement. The standard error of measurement was calculated by dividing the standard deviation of measurement differences by √2.
Results
Descriptive statistics of the children, aged three to five years, with regard to their anthropometric characteristics and ultrasound outcomes are shown in Table 1. Individual SAT thickness sums, including embedded structures, ranged from D Incl = 19.0–122.0 mm and mean thicknesses from d Incl = 2.4–15.3 mm. The t‐test showed no significant group differences between boys and girls in any of the anthropometric measures. The Mann–Whitney U‐test revealed significant differences in SAT values between the sexes, with higher thickness values in girls (d Incl and d Excl: p < 0.01, D Incl and D Excl: p < 0.01) as shown in Table 1. These differences were significant at all eight sites and visible in the fat patterning profiles presented in Figure 2 and in the Table S1. Both boys and girls showed the largest SAT thickness values at the lateral thigh and the smallest thickness values at the erector spinae. The second largest SAT values in girls were found at the lower abdomen, whereas in boys, the distal triceps was the second thickest SAT site. The relationship between anthropometric measures and mean SAT thickness was assessed by calculating the Pearson's product‐moment correlation coefficients. These results are presented in Table 2 for the total sample as well as for boys and girls individually. The results of the multiple regression analysis are presented in Table 3. Body mass alone accounted for 29% (R 2) of the variation in d Incl. When the predictor height was added, body mass and height together accounted for 59% (R 2) of variation in d Incl, In the final model, the combination of all three predictors, body mass, height and sex, explained 66% (R 2) of variation in d Incl. Regarding the descriptive differences in correlation coefficients for d Incl and anthropometric measures in boys and girls, it was further assessed whether the anthropometric characteristics would predict d Incl differently for boys and girls. More specifically, the interaction effects of anthropometric characteristics and sex on d Incl were tested to investigate whether anthropometric characteristics were a better predictor of d Incl for girls than for boys. For this purpose, the variables sex, height, body mass, WC and BMI were transformed to standard z‐scores and the interaction terms of the anthropometric measures and sex were calculated as follows: z‐height*z‐sex, z‐body mass*z‐sex, z‐WC*z‐sex and z‐BMI*z‐sex. Moderation analyses, namely hierarchical linear regression analyses, were performed with d Incl as the outcome variable, the z‐score of a selected anthropometric measure as the predictor, z‐sex as the moderator and the interaction term of moderator and predictor. The moderation effect of sex and any given anthropometric variable were not statistically significant in predicting d Incl.
Table 1.
Description of participating children aged three to five years at inclusion
| Total sample n = 274 | Boys n = 144 | Girls n = 130 | |
|---|---|---|---|
| Age (years) | 4.6 ± 0.7 | 4.6 ± 0.7 | 4.6 ± 0.7 |
| Height (cm) | 108.3 ± 6.6 | 108.8 ± 6.7 | 107.7 ± 6.6 |
| Body mass (kg) | 18.36 ± 3.13† | 18.60 ± 3.14 | 18.09 ± 3.10 |
| Waist circumference (cm) | 51.7 ± 4.0‡ | 51.9 ± 4.1 | 51.4 ± 4.0 |
| BMI | 15.6 ± 1.5† | 15.6 ± 1.5 | 15.5 ± 1.5 |
| BMI percentiles | 50.1 ± 27.6§ | 50.9 ± 27.6 | 49.3 ± 27.7 |
| D Incl (mm) | 46.5 ± 16.3 (19.0–122.0) | 42.6 ± 15.8** (19.0–98.2) | 50.8 ± 15.9** (19.2–122.0) |
| D Excl (mm) | 40.8 ± 15.6 (15.0–113.4) | 36.9 ± 14.9** (15.0–90.6) | 45.1 ± 15.1** (15.3–113.4) |
| d Incl (mm) | 5.8 ± 2.0 (2.4–15.3) | 5.3 ± 2.0** (2.4–12.3) | 6.3 ± 2.0** (2.4–15.3) |
| d Excl (mm) | 5.1 ± 1.9 (1.9–14.2) | 4.6 ± 1.9** (1.9–11.3) | 5.6 ± 1.9** (1.9–14.2) |
BMI = Body mass index; D Incl = Sum of subcutaneous adipose tissue (SAT) thickness at all measured sites including fibrous structures; D Excl = Sum of SAT thickness at all measured sites excluding embedded fibrous structures; d Incl = Mean SAT thickness including embedded fibrous structures; d Excl = Mean SAT excluding embedded fibrous structures.
Values are shown as mean ± standard deviation. BMI percentiles were calculated using German reference values 11.
†n = 272; ‡n = 265; §n = 271; Significant group differences between boys and girls are indicated as **p < 0.01.
Figure 2.
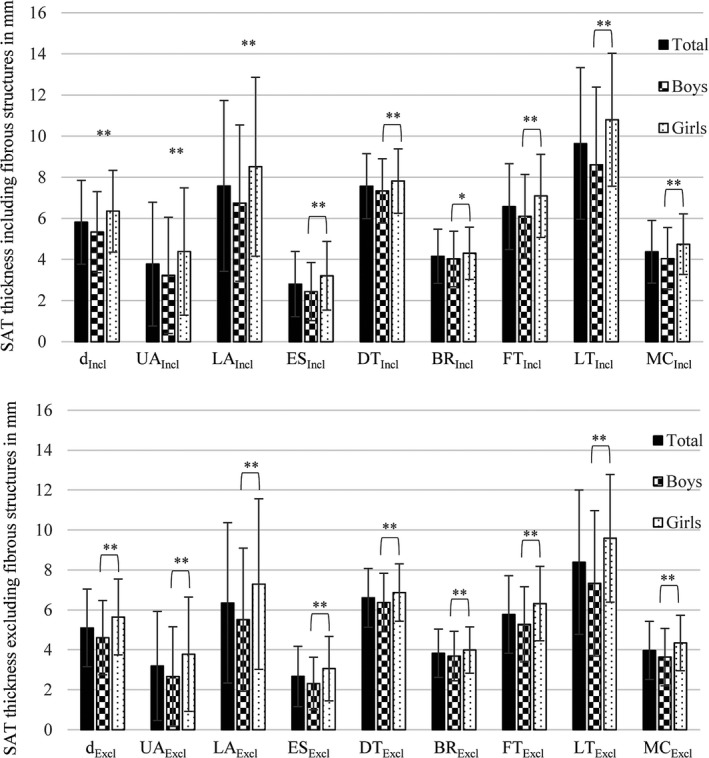
Mean subcutaneous adipose tissue thickness (d) and mean thicknesses at the eight individual sites: upper abdomen (UA), lower abdomen (LA), erector spinae (ES), distal triceps (DT), brachioradialis (BR), front thigh (FT), lateral thigh (LT) and medial calf (MC). Upper image: subcutaneous adipose tissue (SAT) values including (Incl) embedded structures. Lower image: SAT values excluding (Excl) embedded structures. Significant differences between boys and girls indicated as *p < 0.05 and **p < 0.01.
Table 2.
Relationship between anthropometric characteristics and mean subcutaneous adipose tissue thickness (d Incl) based on Pearson's product‐moment correlation coefficients
| d Incl | Height | Body mass† | WC‡ | BMI† | BMI percentiles§ |
|---|---|---|---|---|---|
| Total sample | |||||
| r | 0.123* | 0.543** | 0.654** | 0.754** | 0.686** |
| p | 0.042 | 0.000 | 0.000 | 0.000 | 0.000 |
| Boys | |||||
| r | 0.114 | 0.528** | 0.635** | 0.751** | 0.667** |
| p | 0.175 | 0.000 | 0.000 | 0.000 | 0.000 |
| Girls | |||||
| r | 0.186* | 0.643** | 0.749** | 0.831** | 0.769** |
| p | 0.034 | 0.000 | 0.000 | 0.000 | 0.000 |
BMI = Body mass index; WC = Waist circumference.
BMI percentiles calculated using German reference values 11.
†n = 265; ‡n = 272; §n = 271; Significant correlations between values are indicated as *p ≤ 0.05 and **p < 0.01.
Table 3.
Linear model of predictors of mean subcutaneous adipose tissue thickness (d Incl), with confidence intervals reported in parentheses
| Model | b | SE b | β | p |
|---|---|---|---|---|
| 1 | ||||
| Weight | 0.36 (0.29, 0.42) | 0.03 | 0.54 | 0.000 |
| Constant | −0.71 (−1.94, 0.52) | 0.62 | 0.255 | |
| 2 | ||||
| Weight | 0.85 (0.77 −0.94) | 0.04 | 1.30 | 0.000 |
| Height | −0.29 (−0.33, −0.25) | 0.02 | −0.93 | 0.000 |
| Constant | 21.50 (18.17, 24.77) | 1.68 | 0.000 | |
| 3 | ||||
| Weight | 0.86 (0.78, −0.94) | 0.04 | 1.32 | 0.000 |
| Height | −0.29 (−0.32, −0.25) | 0.02 | −0.92 | 0.000 |
| Sex | 1.15 (0.86, 1.43) | 0.14 | 0.28 | 0.000 |
| Constant | 20.42 (17.43, 23.40) | 1.52 | 0.000 | |
Model 1: R 2 = 0.29; Model 2: R 2 = 0.59; Model 3: R 2 = 0.66.
An intra‐observer reliability analysis of D Incl resulted in an intra‐class correlation coefficient of 0.994 (p < 0.01) with a 95% CI of 0.983 to 0.998. While thickness sums D Incl ranged from 34.83–112.30 mm, the mean difference in D Incl was 0.79 mm, which corresponded to a mean relative difference of 1.3%. This analysis showed that 95% of the differences in D Incl were within −0.44 and 2.03 mm. The standard error of measurement was 1.64 mm.
Discussion
In this study, SAT was measured in 274 preschool children, aged three to five years, using ultrasound in accordance with a standardised protocol for site marking, imaging and image evaluation 23. To our knowledge, this was the first study to evaluate SAT tissue using ultrasound in this standardised manner in children of this age. As an explorative analysis, common anthropometric measures and SAT values were compared between boys and girls. The relationship between anthropometric traits and mean SAT thickness was further assessed to ascertain which of the anthropometric traits could predict mean SAT thickness best.
The results showed that anthropometric measures, such as BMI and WC, were unable to detect sex differences regarding fatness, as was the case with the ultrasound results. Differences in the amount of SAT, with higher values in girls than in boys, were apparent at each individual site as well as in the mean d and the sum of SAT D. This indicates that a significantly different fat patterning profile between boys and girls was already present at this age (Fig. 2).
When assessing and predicting fatness in children, large studies have relied on surrogate measures of adipose tissue and applied methods with limited accuracy 5, 7, 12, 13. It is reasonable to assume that a more sensitive method, such as ultrasound, can distinguish individual and group differences in adipose tissue because of a higher level of accuracy. Similar to our results, Karlsson et al. (2013) found no significant differences between five‐year‐old boys and girls regarding their BMI, WC or waist‐to‐height ratio. They measured total body fat and abdominal fat using dual‐energy X‐ray absorptiometry, which showed significantly higher values of BF% and the percentage of truncal fat in girls than boys. They also used magnetic resonance imaging measurements of the abdomen and these showed no sex differences in SAT, but significantly less visceral adipose tissue in girls than in boys 14. The findings of this study suggest that there is a higher amount of total and mean SAT in girls aged three to five compared with boys and that this also applies to the SAT thickness in the abdominal region.
Assessing the relationship between the anthropometric characteristics and the SAT showed the strongest correlations between BMI, and BMI percentiles, and mean SAT thickness (d Incl), followed by WC and body mass. This suggests that obesity‐related measures also relate to mean SAT thickness in children. Semiz et al. (2007) found that ultrasound measurements of SAT at the abdomen correlated significantly with BMI and WC in obese and non‐obese children with a mean age of 11.5–12.2 years. Significant correlations were also found between measurements of SAT at the triceps and BMI, and WC, but only in non‐obese children 27. Furthermore, ultrasound measurements of SAT at given sites have been found to be related to corresponding segmental fat mass in kilograms as well as BF% measured using dual‐energy X‐ray absorptiometry in young adults aged 18–29 years 22.
In the regression analysis, only raw values such as body mass (kg) and height (cm) rather than BMI or waist‐to‐height ratio were used as predictor variables for d Incl. Due to the high correlation between body mass and WC, WC was withdrawn from the regression model. The final model that was used showed that body mass and height explained 59% of the variation in mean SAT thickness. Adding sex into the model increased the explained variance in d Incl to 66%. Thus, body mass and sex were significant, positive predictors of d Incl, and height was a significant, negative predictor of d Incl. This leads to the assumption that mean SAT thickness will be larger with more body mass, a smaller stature and female sex. A study that assessed the variations in abdominal SAT in 407 children aged seven to 16 years using magnetic resonance imaging, found that body mass explained 78.3% of the variance in abdominal SAT, WC explained 80.4% and BMI explained 88.9% 28.
This study provides information on the distribution of body fat in children aged three to five using ultrasound as a non‐invasive imaging technique and contributes to the assessment of body composition in paediatric studies. However, it is not without limitations. First, although this study was part of a cluster‐randomised trial, the sample used for this analysis was not without selection bias. Second, the applied approach was developed for adults. To obtain accurate imaging of SAT, adjustments had to be made at the medial calf and brachioradialis sites in a few children, so that a parallel alignment of skin, adipose tissue and muscle fascia was assured. This is explained in the Methods section. Third, it was not possible to assess visceral adipose tissue using this protocol. Total adipose tissue includes subcutaneous and visceral adipose tissue, and it is not entirely clear from the literature if children are more likely to store excessive adipose tissue in subcutaneous or visceral compartments 14, 29. However, the results of this study are based on ultrasound thickness measurements of SAT with an image resolution, and thus accuracy, of about 0.1–0.2 mm (at 18 or 9 MHz, respectively), compared to a magnetic resonance imaging total body scan with a pixel size and resolution of about 1.3–2 mm 30. This makes it the most technically accurate method to measure SAT 23. High precision can be achieved when performing measurements in accordance with this standardised protocol. Comparing the sums of SAT D Incl, the intra‐class correlation coefficient (0.994, p < 0.01) and 95% CI (0.983–0.998) indicated excellent intra‐observer reliability: the mean difference in D Incl was 0.79 mm and 95% of differences ranged from −0.44 to 2.03 mm. In comparison, intra‐observer results in a study in normal weight, overweight and obese adults showed that 95% of differences in D Incl ranged from −2.2 to 1.9 mm 24. Inter‐observer results of three observers measuring athletes showed 95% of observer differences from their mean for D Incl were within ±1.1 mm 23. These findings suggest that conducting measurements in this standardised manner provide highly reliable results regardless of age, size and SAT thickness, namely in lean athletes, children and normal or overweight adults.
For routine screening of body fat in paediatric populations, SAT thicknesses measured using ultrasound can be used for comparisons between individuals and groups, as it is performed by the International Society for Advancement in Kinanthropometry when interpreting the sum of skinfolds as an indicator of adiposity 21. In order to assess total body fat based on ultrasound measurements, future studies will have to show whether a reduced number of sites is sufficient for SAT assessments in medical practice, together with how SAT relates to visceral adipose tissue, as it is not measured using this approach. As visceral adipose tissue in children only depicts the smaller part of total adipose tissue on an anatomical level 29, it could be possible to develop models to predict anatomical BF% based on accurate ultrasound measurements of SAT. For this purpose an evaluation must be carried out to determine whether mean SAT thickness d at these eight sites represents all of the superficial adipose tissue and which sites correlate best with BF% assessed on a molecular level, for example using a four‐component model, to enable molecular level BF% assessments based on ultrasound measurements of SAT.
Conclusion
In this study, a non‐invasive imaging technique was applied in a standardised manner to measure SAT in children, aged three to five years. This approach had previously proven effective in measuring SAT in athletes, as well as in normal weight and obese adults 20, 24, 25, but had not yet been used in young children. The results of this analysis showed that reliable measurement of uncompressed SAT was possible in a routine, field setting and that SAT thickness and its patterning differed substantially between boys and girls. Body mass and sex were shown to be significant positive predictors of mean SAT thickness and height was a significant negative predictor of mean SAT thickness.
Currently, ultrasound is the only imaging technique available for assessing body fat distribution in the field. Given the accuracy and reliability of the applied approach, this method is qualified to analyse body fat in various study populations and settings, for example individual and group health assessments, cross‐sectional and longitudinal studies.
Finance
The health survey was, and the programme Join the Healthy Boat continues to be, financed by the Baden‐Württemberg Foundation.
Conflict of interests
The authors have no conflict of interests to declare.
Supporting information
Table S1 Presented data equivalent to Figure 2. Mean subcutaneous adipose tissue thicknesses (in mm) at the eight measured sites including and excluding embedded fibrous structures.
References
- 1. Ahrens W, Pigeot I, Pohlabeln H, De Henauw S, Lissner L, Molnár D, et al. Prevalence of overweight and obesity in European children below the age of 10. Int J Obes (Lond) 2014; 38(Suppl 2): 99–107. [DOI] [PubMed] [Google Scholar]
- 2. de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull 2004; 25(Suppl 1): 27–36. [DOI] [PubMed] [Google Scholar]
- 3. Ahrens W, Bammann K, Siani A, Buchecker K, De Henauw S, Iacoviello L, et al. The IDEFICS cohort: design, characteristics and participation in the baseline survey. Int J Obes (Lond) 2011; 35(Suppl 1): 3–15. [DOI] [PubMed] [Google Scholar]
- 4. Kobel S, Wartha O, Wirt T, Dreyhaupt J, Lämmle C, Friedemann E, et al. Design, implementation, and study protocol of a kindergarten‐based health promotion intervention. Biomed Res Int 2017; 2017: 4347675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paineau D, Chiheb S, Banu I, Valensi P, Fontan JE, Gaudelus J, et al. Comparison of field methods to estimate fat mass in children. Ann Hum Biol 2008; 35: 185–97. [DOI] [PubMed] [Google Scholar]
- 6. Toomey CM, Cremona A, Hughes K, Norton C, Jakeman P. A review of body composition measurement in the assessment of health. Top Clin Nutr 2015; 30: 16–32. [Google Scholar]
- 7. Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child 2006; 91: 612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bammann K, Sioen I, Huybrechts I, Casajus J, Vicente‐Rodriguez G, Cuthill R, et al. The IDEFICS validation study on field methods for assessing physical activity and body composition in children: design and data collection. Int J Obes (Lond) 2011; 35(Suppl 1): 79–87. [DOI] [PubMed] [Google Scholar]
- 9. Fields DA, Goran MI. Body composition techniques and the four‐compartment model in children. J Appl Physiol 2000; 89: 613–20. [DOI] [PubMed] [Google Scholar]
- 10. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kromeyer‐Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V, et al. Perzentile für den Body‐mass‐ Index für das Kindes‐ und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001; 149: 807–18. [Google Scholar]
- 12. Sweeting HN. Measurement and definitions of obesity in childhood and adolescence: a field guide for the uninitiated. Nutr J 2007; 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3‐ to 8‐year‐old children: anthropometry, BIA, and DXA. Obes Res 2004; 12: 1633–40. [DOI] [PubMed] [Google Scholar]
- 14. Karlsson AK, Kullberg J, Stokland E, Allvin K, Gronowitz E, Svensson PA, et al. Measurements of total and regional body composition in preschool children: a comparison of MRI, DXA, and anthropometric data. Obesity (Silver Spring) 2013; 21: 1018–24. [DOI] [PubMed] [Google Scholar]
- 15. Yoo EG. Waist‐to‐height ratio as a screening tool for obesity and cardiometabolic risk. Korean J Pediatr 2016; 59: 425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindsay AR, Hongu N, Spears K, Idris R, Dyrek A, Manore MM. Field assessments for obesity prevention in children and adults: physical activity, fitness, and body composition. J Nutr Educ Behav 2014; 46: 43–53. [DOI] [PubMed] [Google Scholar]
- 17. Nambiar S, Hughes I, Davies PS. Developing waist‐to‐height ratio cut‐offs to define overweight and obesity in children and adolescents. Public Health Nutr 2010; 13: 1566–74. [DOI] [PubMed] [Google Scholar]
- 18. Sijtsma A, Bocca G, L'abée C, Liem ET, Sauer PJ, Corpeleijn E. Waist‐to‐height ratio, waist circumference and BMI as indicators of percentage fat mass and cardiometabolic risk factors in children aged 3‐7 years. Clin Nutr 2014; 33: 311–5. [DOI] [PubMed] [Google Scholar]
- 19. Kriemler S, Puder J, Zahner L, Roth R, Meyer U, Bedogni G. Estimation of percentage body fat in 6‐ to 13‐year‐old children by skinfold thickness, body mass index and waist circumference. Br J Nutr 2010; 104: 1565–72. [DOI] [PubMed] [Google Scholar]
- 20. Müller W, Horn M, Fürhapter‐Rieger A, Kainz P, Kröpfl JM, Maughan RJ, et al. Body composition in sport: a comparison of a novel ultrasound imaging technique to measure subcutaneous fat tissue compared with skinfold measurement. Br J Sports Med 2013; 47: 1028–35. [DOI] [PubMed] [Google Scholar]
- 21. Marfell‐Jones M, Nevill AM, Stewart AD. Anthropometric surrogates for fatness and health In Stewart AD, Sutton L, editors. Body composition in sport, exercise and health, 1st ed. London, UK: Routledge, 2012: 126–46. [Google Scholar]
- 22. Leahy S, Toomey C, McCreesh K, O'Neill C, Jakeman P. Ultrasound measurement of subcutaneous adipose tissue thickness accurately predicts total and segmental body fat of young adults. Ultrasound Med Biol 2012; 38: 28–34. [DOI] [PubMed] [Google Scholar]
- 23. Müller W, Lohman TG, Stewart AD, Maughan RJ, Meyer NL, Sardinha LB, et al. Subcutaneous fat patterning in athletes: selection of appropriate sites and standardisation of a novel ultrasound measurement technique: ad hoc working group on body composition, health and performance, under the auspices of the IOC medical commission. Br J Sports Med 2016; 50: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Störchle P, Müller W, Sengeis M, Ahammer H, Fürhapter‐Rieger A, Bachl N, et al. Standardized ultrasound measurement of subcutaneous fat patterning: high reliability and accuracy in groups ranging from lean to obese. Ultrasound Med Biol 2017; 43: 427–38. [DOI] [PubMed] [Google Scholar]
- 25. Kelso A, Trájer E, Machus K, Treff G, Müller W, Steinacker JM. Assessment of subcutaneous adipose tissue using ultrasound in highly trained junior rowers. Eur J Sport Sci 2017; 17: 576–85. [DOI] [PubMed] [Google Scholar]
- 26. Chan V, Perlas A. Basics of ultrasound imaging In Narouze SN, editor. Atlas of ultrasound‐guided procedures in interventional pain management, 1st ed. New York, NY: Springer, 2011: 13–9. [Google Scholar]
- 27. Semiz S, Ozgören E, Sabir N. Comparison of ultrasonographic and anthropometric methods to assess body fat in childhood obesity. Int J Obes (Lond) 2007; 31: 53–8. [DOI] [PubMed] [Google Scholar]
- 28. Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, et al. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes (Lond) 2006; 30: 23–30. [DOI] [PubMed] [Google Scholar]
- 29. Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross‐sectional whole‐body magnetic resonance imaging study. Nutr Metab (Lond) 2009; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ackland TR, Lohman TG, Sundgot‐Borgen J, Maughan RJ, Meyer NL, Stewart AD, et al. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. medical commission. Sports Med 2012; 42: 227–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Presented data equivalent to Figure 2. Mean subcutaneous adipose tissue thicknesses (in mm) at the eight measured sites including and excluding embedded fibrous structures.


