Summary
Background
Corticosteroids are the most potent drugs for the control of severe equine asthma, but adverse effects limit their chronic systemic administration. Inhaled medications allow for drug delivery directly into the airways, reducing the harmful effects of these drugs.
Objectives
To evaluate the efficacy of inhaled budesonide specifically formulated for the equine use and administered by a novel inhalation device in horses with severe asthma.
Study design
Experimental studies in horses with naturally occurring asthma with cross‐over, randomised, blinded experimental designs.
Methods
In Study 1, budesonide (1800 μg twice daily) administered using a novel Respimat® based inhaler was compared to i.v. dexamethasone (0.04 mg/kg). In Study 2, 3 doses of budesonide (450, 900, and 1800 μg) were compared to oral dexamethasone (0.066 mg/kg). Lung function, bronchoalveolar fluid cytology (Study 1), CBC, serum chemistry, and serum cortisol and adrenocorticotropic hormone (ACTH) values were evaluated.
Results
In Study 1, there was a marked and significant improvement in the lung function of all horses treated with budesonide and dexamethasone. Neutrophil percentages in bronchoalveolar fluid decreased in all horses treated with dexamethasone and in four of six horses treated with budesonide. Serum cortisol and blood ACTH concentrations decreased with both treatments. In Study 2, there was a significant improvement in the lung function with all dosages of budesonide, and the effects of higher dosages were comparable to those of dexamethasone. Dexamethasone and budesonide at the two higher dosages induced a significant decrease of cortisol concentrations.
Main limitations
The Respimat® based inhaler is not currently commercially available.
Conclusions
Administration of budesonide with the Respimat® based inhaler provided dose‐dependent relief of airway obstruction in horses with severe asthma, but also a suppression of serum cortisol.
Keywords: horse, corticosteroid, cortisol, heaves, recurrent airway obstruction
Introduction
Severe equine asthma (also known as heaves or recurrent airway obstruction) is a common and incurable disease of horses associated with stabling and hay feeding. It is characterised by inflammation of the lower airways causing bronchospasm, excess mucus production and airway remodelling. Collectively, these changes lead to exercise intolerance, cough and periods of laboured breathing at rest. Replacing hay with low dust feed and moving horses to a low dust environment, such as pasture, remain the only means for the long‐term control of the disease. Because appropriate environmental changes are often not feasible, severe equine asthma frequently results in a premature retirement of affected horses or euthanasia.
Corticosteroids are the most effective drugs for the treatment of severe equine asthma 1. Systemically administered triamcinolone, isoflupredone, prednisolone and dexamethasone have proven to be efficacious, with the latter being the most potent in comparative studies 2, 3, 4. However, prolonged administration of these drugs have been associated with side effects including adrenal suppression, decreased immune function and, possibly, laminitis 3, 5, 6. Thus, corticosteroids are usually administered systemically only for days to weeks, and relapses of clinical signs are observed soon after drug cessation, in the absence of environmental intervention.
Inhaled medications allow for drug delivery directly into the airways, which limits the dosage required to obtain beneficial effects. In humans, continuous inhaled corticosteroids (ICS) administration has been shown to provide better asthma control than intermittent ICS, and both treatments appear safe 7. While human and equine asthma have many similarities, there are also differences in terms of clinical presentation, pathophysiology and response to therapy 8. However, beclomethasone and fluticasone have been used successfully for the treatment of horses with severe equine asthma, and continuous administration of fluticasone for close to a year was not associated with detectable adverse side effects or alterations of the immune response 9, 10.
The ICS budesonide is commonly administered for the treatment of human asthma. It was recently reported that systemic absorption of budesonide was greater in horses with equine asthma than in controls but its efficacy was not evaluated 11. Therefore, the purpose of this study was to evaluate the efficacy and side effects including cortisol suppression and lameness associated with inhaled budesonide specifically formulated for the equine use and administered by a novel inhalation device in horses with severe asthma.
Materials and methods
Animals
Power analysis revealed that eight horses in a one‐tailed analysis were sufficient to observe a 65% decrease in RL with a 2 weeks treatment with fluticasone 12, another inhaled corticosteroid, with a power of 80% and a significance level at 0.05. Considering that the effects of budesonide on the lung function of asthmatic horses were unknown, it was decided that 12 horses would be initially evaluated.
Twelve adult severely asthmatic horses from our research herd were investigated in two studies. These horses were known to develop lower airway neutrophilic inflammation and laboured breathing at rest when stabled and fed hay. There were eight mares and five geldings of various breeds, 14–25 years of age (18±2.6 years [mean±s.d.]) and weighing 419–556 kg (502±45 kg). Horses had not received corticosteroids for at least 3 weeks before initiation of the studies. Physical examination, upper airway endoscopy, complete blood count and biochemistry profile were performed prior to each study to rule out the presence of concomitant medical conditions. Eight horses participated in both studies, which were performed 18 months apart. Horses were housed in a barn, with daily turnouts. They were fed hay of variable quality and bedded on straw to induce asthma exacerbation and exposure continued throughout the study.
Lung function measurement
Standard lung function measurements were performed as previously described 2, between 08:00 and 10:00. In brief, flow rates and transpulmonary pressure were obtained by the use of a heated pneumotachograph and an oesophageal balloon catheter, respectively. Values of pulmonary resistance (RL) and elastance (EL) were obtained by applying the data to the multiple regression equation for the single compartment model of the lung using a data acquisition and analysis software (Anadat/Labdat)1.
Bronchoalveolar lavage (BAL)
Bronchoalveolar lavages were performed after the lung function measurements, as previously described 12. In brief, horses were sedated and a fibre‐optic flexible endoscope was passed through the nares and directed down into the right lung until wedged in the wall of a bronchus. Two 250 mL boluses of warm sterile isotonic saline solution were instilled and aspirated via the endoscope's biopsy channel. Cytopreparations were stained with a modified Wright's solution. Differential counts were made on 400 cells by a certified clinical pathologist blinded to the treatments.
Cortisol and adrenocorticotropic hormone (ACTH)
Serum cortisol and plasma ACTH were measured using solid‐phase, competitive chemiluminescent enzyme immunoassay (Immulite®)2. The lower limits of detection were 10.2 nmol/L and 2.2 pmol/L for cortisol and ACTH, respectively.
Experimental protocol
Study 1: Inhaled budesonide was compared to i.v. dexamethasone using a randomised, parallel, blinded experimental design. One horse was assigned to the dexamethasone group, as it did not tolerate the inhalation device. Before corticosteroid administration, horses (n = 12) were administered no treatment from day 0 to day 7 and a placebo from day 8 to day 14. The placebo consisted of the budesonide vehicle administered by inhalation (four actuations twice daily). Both budesonide and placebo were administered using an inhalation device developed for horses based on the Respimat® technology. It delivered budesonide and placebo via an equine specific nostril adaptor. Each actuation was initiated at the onset of horse inhalation. At the end of the placebo period, horses were ranked by lung resistance values and randomly divided within that ranking into two treatment groups by tossing a coin. Six horses were given 1800 μg (high dose) of budesonide3 twice daily (eight actuations of 225 μg) at approximately 08:00 and 17:00 for 14 days (Days 15–28). During the same period, the other six horses received 0.04 mg/kg dexamethasone sodium phosphate4 (5 mg/mL) once a day at approximately 08:00 via an intravenous catheter. All horses wore a neck wrap to keep the evaluators blinded to the treatment groups.
Lung function was evaluated weekly until 1 week following the last treatment (Days 0, 7, 14, 28, 35) and after the administration of atropine5 (0.02 mg/kg i.v.) to document reversibility of airway obstruction. Bronchoalveolar lavages were performed prior to enrolment (Day 0), and before and after the treatment phase (Days 14 and 28). Cortisol and ACTH were measured before and after the treatment phase in the morning (Days 14 and 28). Horses were visually observed daily for signs of lameness. When laminitis was suspected based on reluctance to move, increased digital pulses, or pain on hoof testers, the severity of the lameness was assessed using a score previously reported 13.
Study 2: Inhaled budesonide was compared to oral dexamethasone using a complete cross‐over, randomised and blinded experimental design. Eight horses were ranked by lung resistance (RL) values and divided within that ranking in groups of two. Treatment randomisation was performed using the Latin Square model, where all pairs received all four treatments at different times, with a 2‐week washout period between each treatment. The 2‐week treatments consisted of 450 μg (low dose), 900 μg (medium dose), and 1800 μg (high dose) of budesonide administered by inhalation twice daily using a Respimat® based inhaler or 0.066 mg/kg of dexamethasone powder6 administered orally before the morning feeding. This dose was considered equivalent to the dexamethasone dose used in Study 1 based on 61% oral absorption 14. Lung function was measured at baseline and at weekly intervals during and 1 week after each treatment phase (Days 0, 7, 14 and 21). At the completion of the study, lung function was assessed prior to and following administration of atropine (0.02 mg/kg i.v.) to ascertain the reversibility of airway obstruction. Complete blood counts and serum cortisol concentrations were measured on days 0, 14 and 21 of each treatment phase.
Data analysis
In study 1, lung function parameters, BAL fluid percentage of neutrophils, cortisol and ACTH values were analysed using linear mixed models (SAS v.9.4)7 with time, treatment groups (Budesonide versus Dexamethasone) and their interaction as fixed effects and id nested within treatment as a random effect. A priori contrasts were performed to compare treatment mean at each time point and to compare the mean at different time points with the aggregate value of the three pretreatment values in each treatment. For these contrasts, the sequential Benjamini‐Hochberg adjustment procedure was used to adjust alpha level downward. The false discovery rate was set at 5%. Raw P values are reported but changes are described as significant only when the P values are significant after the adjustment of the alpha level 15.
In Study 2, lung function parameters and blood cortisol values were analysed using linear mixed models with time, treatment and their interaction as fixed effects and id, id nested within time and id nested within treatment as random effects. A priori contrasts with sequential Benjamini‐Hochberg alpha‐level adjustments were used to compare pairs of means. Data from seven horses were analysed for treatment period 4, as a horse was removed from the last treatment period because it had a normal lung function and was not included for statistical analysis.
Results
Study 1
One horse in the budesonide group had transient, muco‐purulent nasal discharge between days 25 and 32, which resolved without treatment. Coughing was observed after inhalation in three horses during the placebo period and in four horses during budesonide treatment. No lameness developed during the study period.
There were significant differences across time points for PL, RL and EL (P<0.0001), but not between treatment groups. A significant interaction between treatment group and time was demonstrated for RL (P = 0.02; Fig 1). A priori contrasts revealed that PL, RL, and EL were significantly decreased after 1 and 2 weeks of treatment (P<0.004; Days 21 and 28) when compared to pretreatment values, without significant differences between treatment groups at each time point. However, all horses treated with dexamethasone had values of PL<15 cm H2O, and RL<1 cm H2O/L/s at days 28, while three horses in the budesonides group remained above these values (PL: 20, 23 and 31 cm H2O, RL: 1.2, 1.8, 2.3 cm H2O/L/s). For EL, three and four horses have values <1 cm H2O/L after budesonide and dexamethasone treatment, respectively. One‐week post‐treatment (Day 35), only EL in the budesonide group was significantly lower than pretreatment values (P = 0.0002). Atropine administration resulted in a significant decrease in PL, RL, and EL in both treatment groups (Fig 1).
Figure 1.
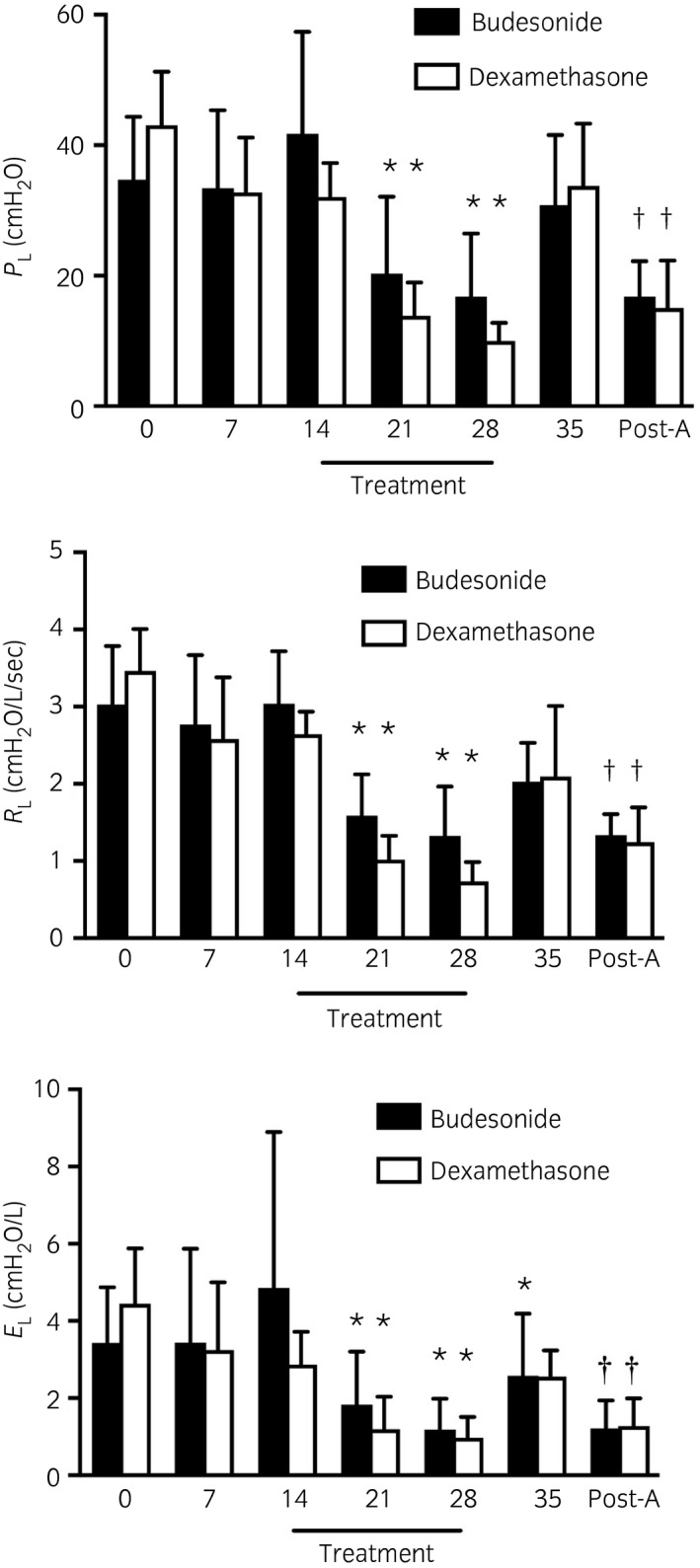
Transpulmonary pressure, pulmonary resistance (RL) and elastance (EL) before and after no treatment (Days 0 and 7), after 1 week of placebo (Day 14), after 1 and 2 weeks of treatment (Days 21 and 28), 1 week after treatment cessation (Day 35) and following atropine administration (Post‐A) in severely asthmatic horses. Black bars represent the budesonide group (n = 6, 1800 μg twice daily [high dose]), white bars represent the dexamethasone group (n = 6, 0.04 mg/kg i.v. once daily). Means with 95% CI. *Significantly different from baseline (mean of pretreatment values). †Significantly different from pre‐atropine administration (Day 35).
There were no significant group differences in the neutrophil percentages in BAL fluid (Fig 2). The neutrophil percentages decreased in all dexamethasone treated horses, and in four of the six budesonide‐treated horses, but this was not statistically significant (P = 0.054). All but two horses maintained neutrophils percentages above 20%. Contrasts showed that the BAL neutrophil percentage was lower on day 28 compared to the mean baseline value in the dexamethasone group (P = 0.002; Fig 2).
Figure 2.
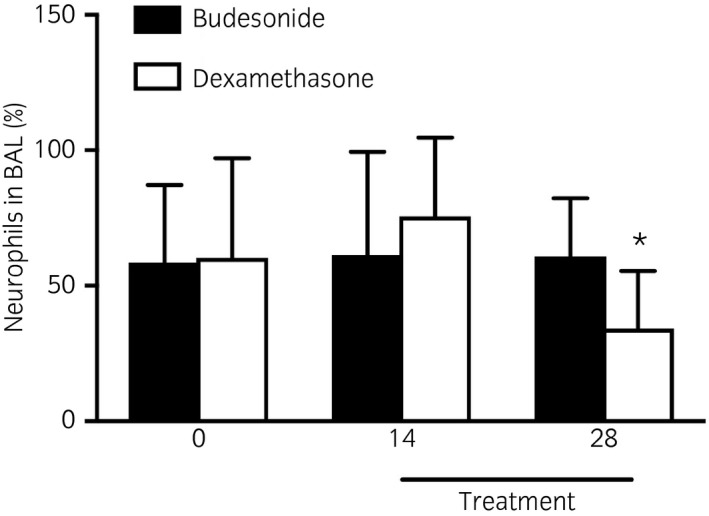
Bronchoalveolar lavage (BAL) fluid neutrophils before (Days 0 and 14) and after treatment (Day 28) with budesonide (n = 6, 1800 μg twice daily, [high dose], black bars) or dexamethasone (n = 6, 0.04 mg/kg i.v. once daily, white bars). Means with 95% CI. *Significantly different from baseline (mean of pretreatment values).
There was a significant decrease in serum cortisol and blood ACTH concentrations with both treatments (Fig 3), with no differences between treatment groups at each time point. Cortisol values were below the detection level in two and four horses of the budesonide and dexamethasone groups, respectively.
Figure 3.
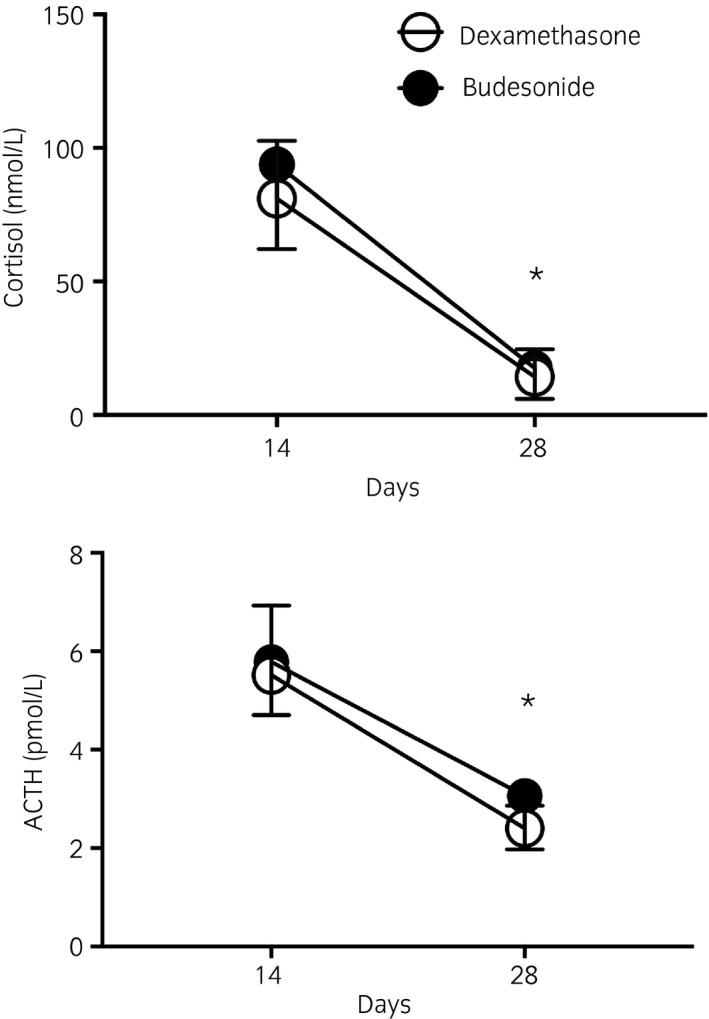
Serum cortisol and plasma ACTH concentrations before (Days 14) and after treatment (Day 28) with budesonide (n = 6, 1800 μg twice daily, [high dose], black circles) or dexamethasone (n = 6, 0.04 mg/kg i.v. once daily, white circles). Means with 95% CI. *Significantly different from baseline in both treatment groups.
Study 2
While receiving budesonide during the first treatment period, one horse exhibited signs consistent with mild colic, including dry manure and increased time spent laying down. This horse also developed transient increased rectal temperature (39.1–40.1°C on five separate days) and tachycardia (60–80 beats per min on three separate days) during the first two treatment periods (900 and 450 μg BID, respectively). Signs of colic resolved after administration of mineral oil (4.5 L mineral oil via nasogastric intubation) and intravenous fluids (15 L Lactated Ringer's Solution over a 3‐h period). During episodes of increased rectal temperature, the horse was treated with antimicrobials (Procaine penicillin 25 mg/kg i.m. once, and then ceftiofur 2 mg/kg i.m. or i.v. BID for 8 days), and phenylbutazone (1 g i.v. or per os for 2 days). Another horse receiving budesonide presented with episodes of increased rectal temperature (38.8–39.3°C on three separate days during the 900 and 1800 μg BID, and during a wash‐out period) which were controlled with phenylbutazone administration (1 g, per os, once). Each time, CBC and clinical examination were not supportive of pneumonia. Cough and/or sneeze following inhalation of budesonide were observed in eight of the nine horses. Three of these horses also coughed regularly throughout the day and two of them coughed following placebo administration. No lameness developed during the study period.
Treatment order did not significantly influence the lung function measurements and there were no differences in PL, RL and EL between treatment groups at baseline (Day 0). There was a significant decrease in PL, RL and EL after 7 and 14 days of treatment with dexamethasone and all dosages of budesonide. A dose‐dependent effect was observed with budesonide (Fig 4), however, and a residual beneficial effect 7 days after treatment cessation (Day 21) was present only with dexamethasone (Fig 4). There was a significant improvement in lung function parameters (PL, RL and EL) with atropine in all horses (Fig 5).
Figure 4.
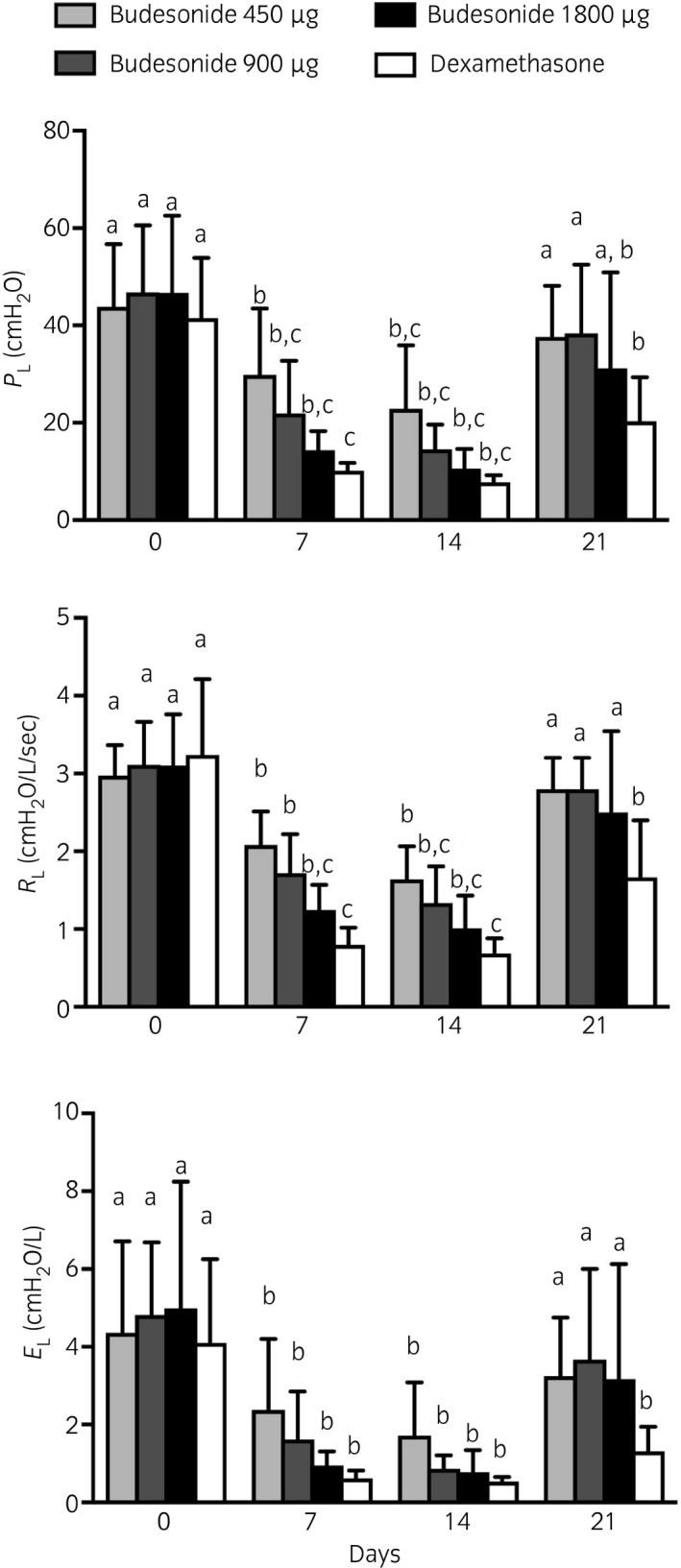
Transpulmonary pressure (PL), pulmonary resistance (RL) and elastance (EL) before treatment administration (Day 0), after 1 and 2 weeks of treatment (Days 7 and 14), and 1 week after treatment cessation (Day 21). All horses received budesonide 450 μg (low dose, light grey), 900 μg (medium dose, dark grey), 1800 μg (high dose, black bars) by inhalation twice a day and dexamethasone 0.04 mg/kg orally once a day (white bars) in a Latin Square Model. Means with 95% CI. Columns that do not share at least one letter are significantly different.
Figure 5.
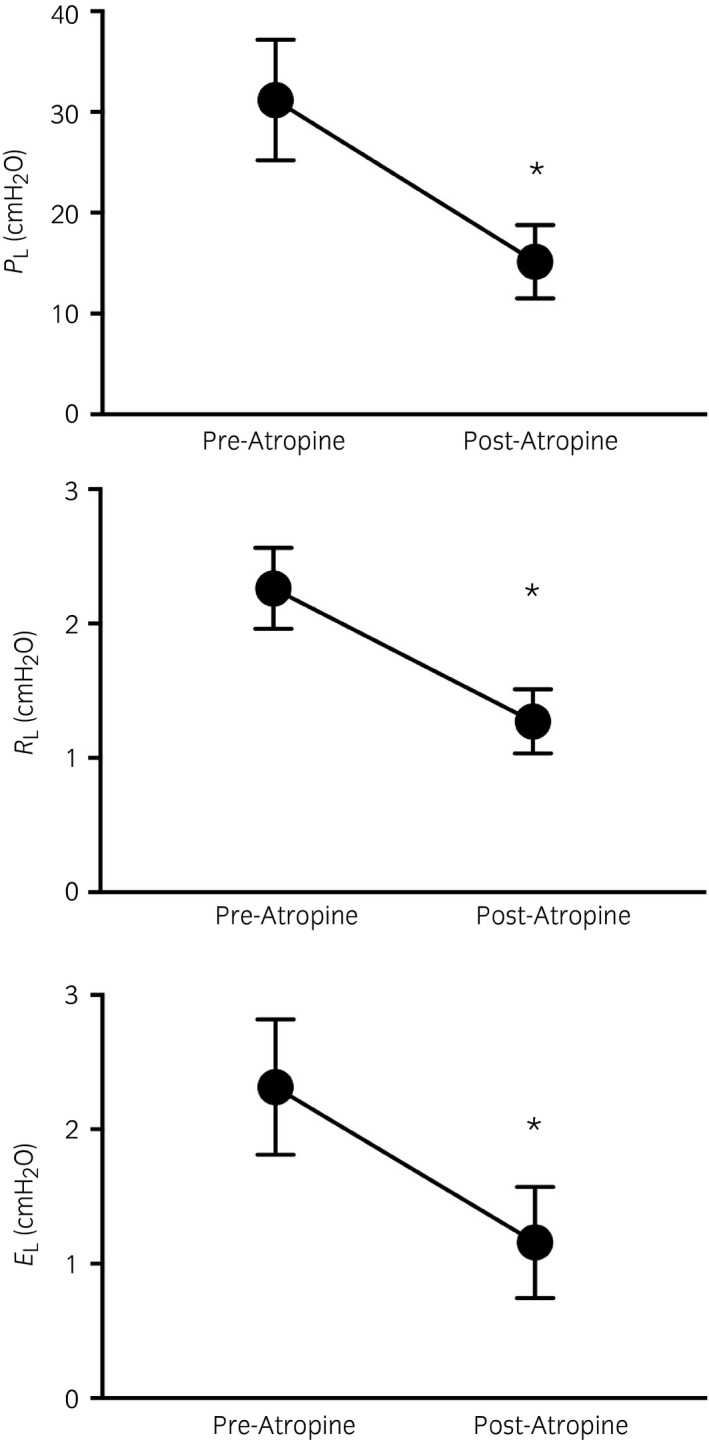
Transpulmonary pressure, pulmonary resistance (RL) and elastance (EL) before and after atropine administration. Means with 95% CI. *Significantly different from pre atropine value.
Dexamethasone and budesonide at the two higher dosages (900 and 1800 μg) induced a significant decrease of serum cortisol concentrations after 2 weeks of administrations, with no residual suppression 1 week after cessation of treatment (Fig 6).
Figure 6.
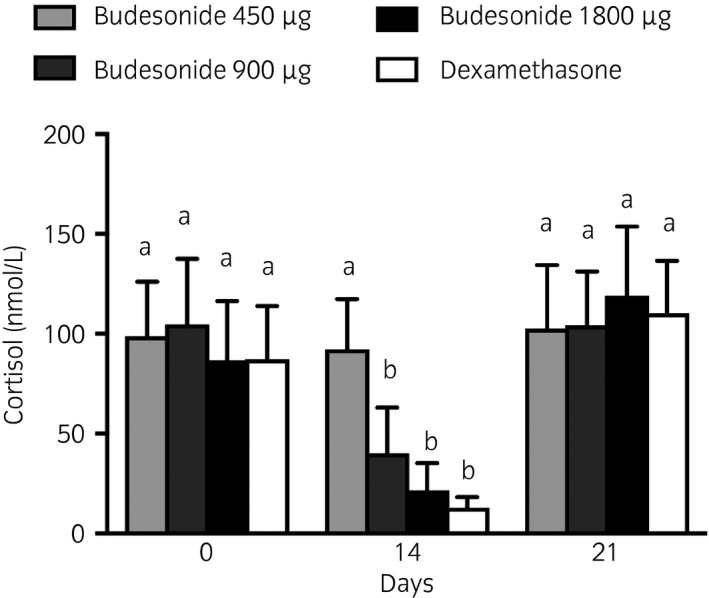
Serum cortisol concentration before treatment administration (Day 0), after 1 and 2 weeks of treatment (Days 7 and 14), and 1 week after treatment cessation Day 21). All horses received budesonide 450 μg (low dose, light grey), 900 μg (medium dose, dark grey), 1800 μg (high dose, black bars) by inhalation twice a day and dexamethasone 0.066 mg/kg orally once a day (white bars) in a Latin Square Model. Means with 95% CI. Columns that do not share at least one letter are significantly different.
Discussion
Results of the current study indicate that inhaled budesonide, when administered using a new equine device based on the Respimat® technology, is effective at controlling airway obstruction in severe equine asthma. At higher dosages, the improvement in lung function was of a similar magnitude as observed with systemically administered dexamethasone. The dose titration study also indicates that the effectiveness and cortisol suppression with budesonide is dose dependent, and of a similar magnitude as observed with dexamethasone at equipotent concentrations.
Corticosteroids are the most effective drugs for the treatment of equine asthma, with dexamethasone having the greatest potency 2, 4, 16, 17. However, because of the side effects that are feared with their use, corticosteroids are usually administered systemically only for short periods (days to weeks), with clinical signs reappearing quickly after treatment cessation when the environment is not improved concurrently. In human asthmatic patients, corticosteroids administered by inhalation are associated with improved toxicity profiles and with lesser side‐effects than when administered systemically, allowing for their chronic administration. To date, studies of the comparative efficacy and toxicity of inhaled vs. systemic corticosteroids in horses with asthma are few. Beclomethasone 18, 19, 20 and fluticasone 21, 22, 23, 24 have been shown to be efficacious at controlling the airway obstruction in severe equine asthma. Beclomethasone diproprionate (1320 μg, q 12 h) administered by inhalation significantly improved the lung function of horses with severe asthma, although the magnitude of the response was less than that of dexamethasone 19. Surprisingly, however, a low‐dose (500 μg) of beclomethasone was reported to be as effective as higher dose (1500 μg) 25. In contrast, the present study demonstrated dose‐dependent improvements in lung function when inhaled budesonide was administered at low, medium and high doses, with only the highest dose resulting in changes comparable to that of dexamethasone. Our findings also agree with previous reports indicating that the degree of improvement with both inhaled and systemically administered corticosteroids is not predicted by the degree of airway obstruction. Indeed, some of the best responders in the present study were horses with marked airway obstruction (data not shown) and does not support the concept that less severely affected horses are more likely to respond better to the medication. Also, little residual effects were noted after treatment cessation, irrespective of the dose administered.
Corticosteroids are used in asthma due to their potent anti‐inflammatory effects. However, while improving the clinical signs and lung function, these drugs have generally been found to have mild to no benefit at controlling the neutrophilic inflammation in equine asthma 12, 26, 27, unless combined with antigen avoidance strategies 24, 28. In agreement with these findings, when compared to baseline values, 402 horses and 582 horses would have been required in the budesonide group to identify significant differences in the neutrophil percentages at Day 14 and Day 28, respectively, with a power of 80% and alpha set at 0.05. Also, while dexamethasone was associated with a mild but significant decrease in airway neutrophils in the present study, only the lung function had normalised. The poor response of neutrophilic inflammation to corticosteroids is not limited to horses, as similar findings have also been reported in patients with neutrophilic asthma 29. While it is generally assumed that this is the result of an intrinsic corticoresistance of these cells, it was recently shown that equine and human circulating neutrophils are at least as sensitive to corticosteroids as other blood leukocytes 30.
It is expected that inhaled corticosteroids lessen the side effects associated with their systemic administration when used for the treatment of lung diseases. Nevertheless, inhaled corticosteroids will reach the systemic circulation, and their systemic effects will be primarily determined by their absorption by the pulmonary and gastrointestinal tracts, but also by their potency, systemic half‐life, device efficiency, receptor affinity and pulmonary residency time 31, 32. The systemic side effects of inhaled corticosteroids have been primarily assessed by evaluating the suppression of endogenous cortisol production by an inhibitory feedback mechanism on the hypothalamic‐pituitary–adrenal axis. We therefore compared the effects of budesonide to those of dexamethasone on serum cortisol concentrations and found that dosages required for clinical efficacy were associated with cortisol suppression. These findings contrast with those in humans, where adrenal suppression is usually not observed within the licensed dose range 31. However, our findings are in agreement with reports of adrenal suppression developing at dosages required to improve lung function in severe equine asthma with inhaled beclomethasone and fluticasone when used as solitary therapy 16, 33. Nevertheless, the clinical relevance of decreased blood cortisol in horses has yet to be determined, especially when the adrenal gland responsiveness to ACTH administration is preserved 16. Finally, no significant side effects could be directly linked to the systemic absorption of budesonide in this study. However, two horses developed episodes of increased rectal temperature while being treated. It is unknown whether this was a fever secondary bacterial infection, or hyperthermia due to disturbed thermoregulation. Lastly, budesonide administration (and placebo) elicited cough in some horses, which may have important implications for lung deposition as well as long‐term client and animal compliance. The sample size was not large enough to associate the cough to the vehicle, the drug, the device or simply to the underlying disease.
The choice of inhalation device contributes to drug efficacy. While the lung deposition of budesonide with the Respimat® has not been evaluated, previous studies in horses have reported variable lung deposition depending on the device used and the severity of the airway obstruction 34, 35. Drug administration with the equine Respimat® based inhaler was well tolerated by horses. This novel inhalation device for horses was developed based on the Respimat® Soft Mist™ Inhaler technology used in human 36. This pocket sized device generates an aerosol of inhalable droplets without electricity in a standard, reproducible, patient independent way. The improvement in EL was especially marked, suggesting a deposition of the drug in the most distal airways where most of the pathology is found in this disease 37. The high fine particle fraction produced with the Respimat® SMI™‐technology allied with the low velocity and long generation time of the aerosol translate into a higher fraction of the emitted dose being deposited in the lungs compared with aerosols from pressurised dose inhalers and dry powder inhalers 36. This may be of an advantage over metered dose inhalers as it improves the delivery of the drug in the lower airways by decreasing drug deposition in the upper airways.
Conclusions
Administration of budesonide with the Respimat® based inhaler provided dose‐dependent symptomatic relief of airway obstruction. The higher administered dose of budesonide provided improvement in lung function but also resulted in suppression of serum cortisol comparable to those observed with dexamethasone treatment.
Authors’ declaration of interests
B. Albrecht is employed by Boehringer Ingelheim Vetmedica GmbH, Germany. No other competing interests have been declared.
Ethical animal research
The experimental protocols of this study were approved by the Animal Care Committee of the Université de Montreal (09‐Rech‐1466 and 09‐Rech‐1490).
Source of funding
Funding was provided by Boehringer Ingelheim Vetmedica GmbH.
Authorship
J.P. Lavoie contributed to study design, data collection, data analysis and interpretation, and preparation of the manuscript. M. Leclerc contributed to data collection, data analysis and interpretation, and preparation of the manuscript. K. Lemos and C. Bourzac contributed to data collection. N. Rodrigues and J. Lefebvre‐Lavoie contributed to data collection, data analysis and interpretation and preparation of the manuscript. G. Beauchamp contributed to study design, data analysis and preparation of the manuscript. B. Albrecht contributed to study design, and preparation of the manuscript. All authors gave their final approval of the manuscripit.
Acknowledgements
The authors would like to thank E. Gélinas, K. Théroux, E. Laflamme, M. Bélanger, and A. Lavoie‐Lamoureux for their contributions.
Manufacturers' addresses
RHT‐Infodat, Montréal, Quebec, Canada.
Siemens Healthcare Diagnostics Inc., Deerfield, Illinois, USA.
Boehringer Ingelheim Vetmedica GmbH, Ingleheim am Rhein, Germany.
Vetoquinol, Lavaltrie, Quebec, Canada.
Rafter 8 Products, Calgary, Canada.
Dominion Veterinary Laboratories Ltd., Winnipeg, Canada.
SAS Institute, Cary, North Carolina, USA.
References
- 1. Leguillette, R. (2003) Recurrent airway obstruction–heaves. Vet. Clin. N. Am.: Equine Pract. 19, 63‐86, vi. [DOI] [PubMed] [Google Scholar]
- 2. Picandet, V. , Leguillette, R. and Lavoie, J.P. (2003) Comparison of efficacy and tolerability of isoflupredone and dexamethasone in the treatment of horses affected with recurrent airway obstruction (‘heaves’). Equine Vet. J. 35, 419‐424. [DOI] [PubMed] [Google Scholar]
- 3. Lapointe, J.M. , Lavoie, J.P. and Vrins, A.A. (1993) Effects of triamcinolone acetonide on pulmonary function and bronchoalveolar lavage cytologic features in horses with chronic obstructive pulmonary disease. Am. J. Vet. Res. 54, 1310‐1316. [PubMed] [Google Scholar]
- 4. Leclere, M. , Lefebvre‐Lavoie, J. , Beauchamp, G. and Lavoie, J.P. (2010) Efficacy of oral prednisolone and dexamethasone in horses with recurrent airway obstruction in the presence of continuous antigen exposure. Equine Vet. J. 42, 316‐321. [DOI] [PubMed] [Google Scholar]
- 5. Ryu, S.H. , Kim, B.S. , Lee, C.W. , Yoon, J. and Lee, Y.L. (2004) Glucocorticoid‐induced laminitis with hepatopathy in a Thoroughbred filly. J. Vet. Sci. 5, 271‐274. [PubMed] [Google Scholar]
- 6. Slack, J. , Risdahl, J.M. , Valberg, S.J. , Murphy, M.J. , Schram, B.R. and Lunn, D.P. (2000) Effects of dexamethasone on development of immunoglobulin G subclass responses following vaccination of horses. Am. J. Vet. Res. 61, 1530‐1533. [DOI] [PubMed] [Google Scholar]
- 7. Chauhan, B.F. , Chartrand, C. and Ducharme, F.M. (2013) Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database Syst. Rev. 2, CD009611. [DOI] [PubMed] [Google Scholar]
- 8. Bullone, M. and Lavoie, J.P. (2015) Asthma “of horses and men”–how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 66, 97‐105. [DOI] [PubMed] [Google Scholar]
- 9. Calzetta, L. , Roncada, P. , di Cave, D. , Bonizzi, L. , Urbani, A. , Pistocchini, E. , Rogliani, P. and Matera, M.G. (2017) Pharmacological treatments in asthma‐affected horses: a pair‐wise and network meta‐analysis. Equine Vet. J. 49, 710‐717. [DOI] [PubMed] [Google Scholar]
- 10. Dauvillier, J. , Felippe, M.J. , Lunn, D.P. , Lavoie‐Lamoureux, A. , Leclere, M. , Beauchamp, G. and Lavoie, J.P. (2011) Effect of long‐term fluticasone treatment on immune function in horses with heaves. J. Vet. Intern. Med. 25, 549‐557. [DOI] [PubMed] [Google Scholar]
- 11. Barton, A.K. , Heinemann, H. , Schenk, I. , Machnik, M. and Gehlen, H. (2017) Influence of respiratory tract disease and mode of inhalation on detectability of budesonide in equine urine and plasma. Am. J. Vet. Res. 78, 244‐250. [DOI] [PubMed] [Google Scholar]
- 12. Lavoie, J.P. , Leguillette, R. , Pasloske, K. , Charette, L. , Sawyer, N. , Guay, D. , Murphy, T. and Hickey, G.J. (2002) Comparison of effects of dexamethasone and the leukotriene D4 receptor antagonist L‐708,738 on lung function and airway cytologic findings in horses with recurrent airway obstruction. Am. J. Vet. Res. 63, 579‐585. [DOI] [PubMed] [Google Scholar]
- 13. Obel, N. (1948) Studies on the Histopathology of Acute Laminitis [dissertation], Almqvist & Wiksells Boltryckeri, AB, Uppsala: pp 95. [Google Scholar]
- 14. Cunningham, F.E. , Rogers, S. , Fischer, J.H. and Jensen, R.C. (1996) The pharmacokinetics of dexamethasone in the thoroughbred racehorse. J. Vet. Pharmacol. Ther. 19, 68‐71. [DOI] [PubMed] [Google Scholar]
- 15. Benjamini, Y. and Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 57, 289‐300. [Google Scholar]
- 16. Rush, B.R. , Worster, A.A. , Flaminio, M.J. , Matson, C.J. and Hakala, J.E. (1998) Alteration in adrenocortical function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am. J. Vet. Res. 59, 1044‐1047. [PubMed] [Google Scholar]
- 17. Courouce‐Malblanc, A. , Fortier, G. , Pronost, S. , Siliart, B. and Brachet, G. (2008) Comparison of prednisolone and dexamethasone effects in the presence of environmental control in heaves‐affected horses. Vet. J. 175, 227‐233. [DOI] [PubMed] [Google Scholar]
- 18. Ammann, V.J. , Vrins, A.A. and Lavoie, J.P. (1998) Effects of inhaled beclomethasone dipropionate on respiratory function in horses with chronic obstructive pulmonary disease (COPD). Equine Vet. J. 30, 152‐157. [DOI] [PubMed] [Google Scholar]
- 19. Rush, B.R. , Raub, E.S. , Rhoads, W.S. , Flaminio, M.J. , Matson, C.J. , Hakala, J.E. and Gillespie, J.R. (1998) Pulmonary function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am. J. Vet. Res. 59, 1039‐1043. [PubMed] [Google Scholar]
- 20. Couetil, L.L. , Art, T. , de Moffarts, B. , Becker, M. , Melotte, D. , Jaspar, F. , Bureau, F. and Lekeux, P. (2006) Effect of beclomethasone dipropionate and dexamethasone isonicotinate on lung function, bronchoalveolar lavage fluid cytology, and transcription factor expression in airways of horses with recurrent airway obstruction. J. Vet. Intern. Med. 20, 399‐406. [DOI] [PubMed] [Google Scholar]
- 21. Viel, L. (1999) Therapeutic efficacy of inhaled fluticasone propionate in horses with chronic obstructive pulmonary disease. Proc. Am. Ass. Equine Practnrs 45, 306‐307. [Google Scholar]
- 22. Couetil, L.L. , Chilcoat, C.D. , DeNicola, D.B. , Clark, S.P. , Glickman, N.W. and Glickman, L.T. (2005) Randomized, controlled study of inhaled fluticasone propionate, oral administration of prednisone, and environmental management of horses with recurrent airway obstruction. Am. J. Vet. Res. 66, 1665‐1674. [DOI] [PubMed] [Google Scholar]
- 23. Robinson, N.E. , Berney, C. , Behan, A. and Derksen, F.J. (2009) Fluticasone propionate aerosol is more effective for prevention than treatment of recurrent airway obstruction. J. Vet. Intern. Med. 23, 1247‐1253. [DOI] [PubMed] [Google Scholar]
- 24. Leclere, M. , Lavoie‐Lamoureux, A. , Joubert, P. , Relave, F. , Setlakwe, E.L. , Beauchamp, G. , Couture, C. , Martin, J.G. and Lavoie, J.P. (2012) Corticosteroids and antigen avoidance decrease airway smooth muscle mass in an equine asthma model. Am. J. Respir. Cell Mol. Biol. 47, 589‐596. [DOI] [PubMed] [Google Scholar]
- 25. Rush, B.R. , Raub, E.S. , Thomsen, M.M. , Davis, E.G. , Matson, C.J. and Hakala, J.E. (2000) Pulmonary function and adrenal gland suppression with incremental doses of aerosolized beclomethasone dipropionate in horses with recurrent airway obstruction. J. Am. Vet. Med. Ass. 217, 359‐364. [DOI] [PubMed] [Google Scholar]
- 26. Rush, B.R. , Flaminio, M.J. , Matson, C.J. , Hakala, J.E. and Shuman, W. (1998) Cytologic evaluation of bronchoalveolar lavage fluid from horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am. J. Vet. Res. 59, 1033‐1038. [PubMed] [Google Scholar]
- 27. Robinson, N.E. , Jackson, C. , Jefcoat, A. , Berney, C. , Peroni, D. and Derksen, F.J. (2002) Efficacy of three corticosteroids for the treatment of heaves. Equine Vet. J. 34, 17‐22. [DOI] [PubMed] [Google Scholar]
- 28. Giguere, S. , Viel, L. , Lee, E. , MacKay, R.J. , Hernandez, J. and Franchini, M. (2002) Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate. Vet. Immunol. Immunopathol. 85, 147‐158. [DOI] [PubMed] [Google Scholar]
- 29. Green, R.H. , Brightling, C.E. , Woltmann, G. , Parker, D. , Wardlaw, A.J. and Pavord, I.D. (2002) Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 57, 875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirsch, G. , Lavoie‐Lamoureux, A. , Beauchamp, G. and Lavoie, J.P. (2012) Neutrophils are not less sensitive than other blood leukocytes to the genomic effects of glucocorticoids. PLoS One 7, e44606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wales, D. , Makker, H. , Kane, J. , McDowell, P. and O'Driscoll, B.R. (1999) Systemic bioavailability and potency of high‐dose inhaled corticosteroids: a comparison of four inhaler devices and three drugs in healthy adult volunteers. Chest 115, 1278‐1284. [DOI] [PubMed] [Google Scholar]
- 32. Daley‐Yates, P.T. (2015) Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br. J. Clin. Pharmacol. 80, 372‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munoz, T. , Leclere, M. , Jean, D. and Lavoie, J.P. (2015) Serum cortisol concentration in horses with heaves treated with fluticasone proprionate over a 1 year period. Res. Vet. Sci. 98, 112‐114. [DOI] [PubMed] [Google Scholar]
- 34. Mazan, M.R. , Lascola, K. , Bruns, S.J. and Hoffman, A.M. (2014) Use of a novel one‐nostril mask‐spacer device to evaluate airway hyperresponsiveness (AHR) in horses after chronic administration of albuterol. Can. J. Vet. Res. 78, 214‐220. [PMC free article] [PubMed] [Google Scholar]
- 35. Rush, B.R. , Hoskinson, J.J. , Davis, E.G. , Matson, C.J. and Hakala, J.E. (1999) Pulmonary distribution of aerosolized technetium Tc 99 m pentetate after administration of a single dose of aerosolized albuterol sulfate in horses with recurrent airway obstruction. Am. J. Vet. Res. 60, 764‐769. [PubMed] [Google Scholar]
- 36. Dalby, R.N. , Eicher, J. and Zierenberg, B. (2011) Development of Respimat((R)) Soft Mist Inhaler and its clinical utility in respiratory disorders. Med. Devices (Auckl.) 4, 145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herszberg, B. , Ramos‐Barbon, D. , Tamaoka, M. , Martin, J.G. and Lavoie, J.P. (2006) Heaves, an asthma‐like equine disease, involves airway smooth muscle remodeling. J. Allergy Clin. Immunol. 118, 382‐388. [DOI] [PubMed] [Google Scholar]


