Figure 1.
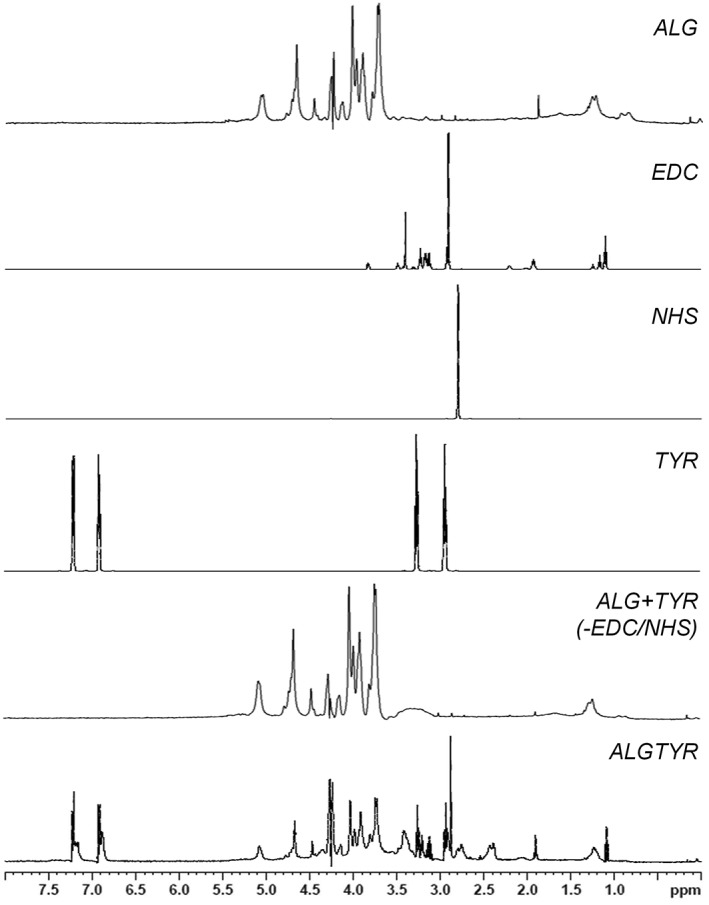
The functionalization of alginate hydrogels (ALG) with tyramine (TYR) using the carbodiimide chemistry (EDC, NHS) was confirmed by the structural elucidation via 1H NMR spectroscopy. Besides the typical homo‐ and heteropolymeric block fractions of alginates consisting of mannuronate and guluronate, the aromatic protons of the introduced tyramines phenolic group (6.88–7.23 ppm) were present within the tyramine‐alginate (ALGTYR) spectra. Without using EDC/NHS, tyramine did not bind to ALG excluding an unspecific reaction (ALG + TYR (−EDC/NHS)).
