ABSTRACT
Negative-pressure pulmonary edema (NPPE)-related diffuse alveolar hemorrhage (DAH) is an underdiagnosed clinical entity seen with alveolar capillary damage. The pathophysiology of type I NPPE is generation of a negative pleural pressure against an upper airway obstruction. We suspect this process was facilitated by preexisting alveolar damage with smoking and administration of the irritating and coagulopathic inhaled anesthetic sevoflurane. We present a case of a healthy 31-year-old man who developed postoperative hemoptysis, diffuse ground-glass opacity and infiltrates on computed tomography (CT) of the chest, anemia, and hypoxic respiratory failure. A diagnosis of DAH was made and a serologic workup for systemic disorders including vasculitis and connective tissue diseases was negative. The patient rapidly improved with supportive care and had complete resolution of his bilateral infiltrates on repeat chest x-ray two weeks later. Our literature review identified three cases of DAH in the setting of sevoflurane administration. Our case illustrates the importance of including NPPE-related DAH on the differential of post-operative hemoptysis, especially in association with sevoflurane administration and a history of cigarette smoking.
KEYWORDS: Sevoflurane, negative-pressure pulmonary edema, cigarette smoking, hemoptysis, diffuse alveolar hemorrhage
1. Introduction
Diffuse alveolar hemorrhage (DAH) is an accumulation of intraalveolar red blood cells (RBCs) originating from damaged alveolar microvasculature. The cardinal manifestation of DAH is hemoptysis and may be dramatic in presentation [1]. Though hemoptysis is mostly self-limiting, a fraction may be severe or massive warranting aggressive work-up and treatment. Prompt recognition of the causes of hemoptysis is vital to avoiding fatal complications [2].
DAH is associated with a spectrum of disorders ranging from localized damage by inhalation injury to systemic disorders including vasculitis or connective tissue diseases [1]. Though rare, negative-pressure pulmonary edema (NPPE)-related DAH is frequently underdiagnosed and needs to be suspected with tonic-clonic seizures or general anesthesia [3]. NPPE is a well-recognized post-operative clinical entity and is typically described in association with DAH in the setting of acute upper airway obstructions [4].
We present a case of a patient with post-operative DAH seen in association with sevoflurane administration contributed to by cigarette smoking.
2. Case description
A healthy 31-year-old man employed as a landscaper with no medical or surgical history was referred from a partner hospital with a nail lodged in the distal humerus from an automatic nail gun. An x-ray reported a nondisplaced vertical linear fracture in the distal humeral diaphysis extending into the proximal metaphysis. Orthopedic surgery scheduled the patient for foreign body removal later that day. The patient had already been administered intravenous (IV) ampicillin-sulbactam, IV ondansetron, and IV hydromorphone. He was administered additional IV ampicillin-sulbactam and cefazolin prior to surgery. His preoperative physical examination and laboratory values were unremarkable.
General anesthesia was induced with fentanyl (50 µg) and propofol (300 mg). A laryngeal mask airway was placed without complication and anesthesia was maintained with inspired sevoflurane range of 1.5 to 3.5%. The patient maintained spontaneous respirations with oxygen saturation > 99%. At the conclusion of the 30-minute case, he was transferred to the post-anesthesia care unit (PACU) with minimal post-operative blood loss. In the PACU, he was somnolent with supraglottic airway obstruction needing jaw lift assistance. His oxygen saturation improved rapidly from the 70% range to > 90% with supplemental oxygen and nasopharyngeal airway placement.
Six hours postoperatively, he was observed to have blood-tinged sputum. Twelve hours postoperatively, he had a persistent cough productive of three tablespoons of bloody sputum. He had no additional complaints. He denied any history of hemoptysis, pulmonary disease, or cough prior to surgery. He smoked one cigarette per day without any history of alcohol or substance abuse. His vitals were stable and his respiratory rate was at baseline. A chest x-ray (CXR) reported patchy bilateral infiltrates. (Figure 1(a)). A follow-up chest computed tomography (CT) reported geographic areas of ground glass opacity involving all pulmonary lobes with consolidative changes in the posterior upper lobes. (Figure 2) A repeat complete blood count reported a hemoglobin drop from 14.2 g per dL to 12.7 g per dL (reference range: 4.50–6.10 gram/dL). The patient was transferred to the pulmonary critical care service.
Figure 1.
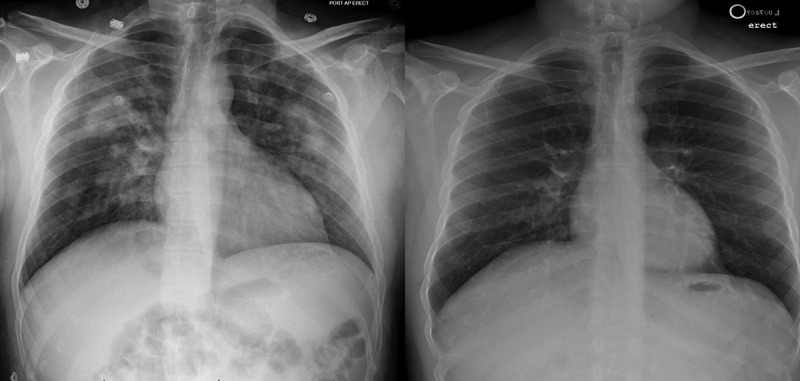
Chest x rays from admission showing bilateral interstitial infiltrates (1A) and from 2 weeks showing complete resolution (1B).
Figure 2.
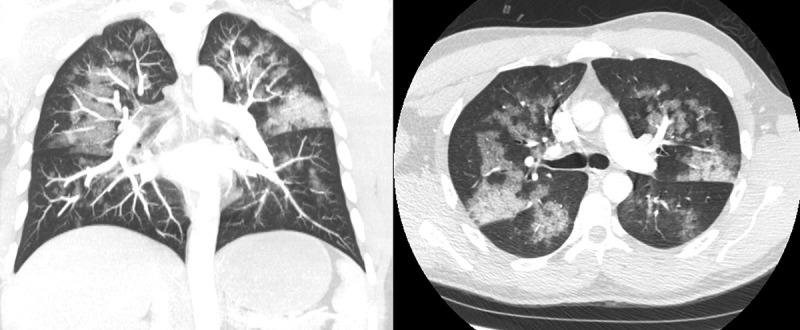
Chest computed tomography (CT) showing diffuse ground glass opacities.
On the constellation of findings including hemoptysis, characteristic diffuse ground-glass opacity and infiltrates, anemia, and hypoxemic respiratory failure, our patient was diagnosed with DAH. An exclusion of differential diagnoses was notable for negative 1:40 anti-nuclear antibody (ANA) (reference range: negative 1:40), negative < 0.3 anti-neutrophilic cytoplasmic antibody (ANCA) (reference range: negative < 3.4U/mL), negative < 1.9 IgG glomerular basement membrane antibody (GBM) (reference range: negative <6.9 U/mL), negative 2 GPL IgG cardiolipin antibody (reference range: negative < 14 GPL), and normal 3 mm per hour sedimentation rate (reference range: 0–15 mm/hr). His partial thromboplastin time was 29 seconds (reference range: 23–34 seconds) and international normalized ratio was 1.2 (reference range: 0.9–1.1). He was managed supportively and oxygen requirements gradual decreased. His deep venous thrombosis prophylaxis was held and additional treatment with bronchoscopy and steroids was deferred as the patient rapidly improved. He was discharged in two days and a repeat CXR in two weeks confirmed that the previous bilateral infiltrates had resolved. (Figure 1(b))
3. Discussion
DAH is a syndrome with findings including hemoptysis, anemia, diffuse radiographic pulmonary infiltrates, and hypoxemic respiratory failure. Multiple systemic vasculitides and connective tissue diseases are associated and directed serologic testing is often necessary. Some commonly associated disorders include systemic lupus erythematosus, anti-glomerular basement membrane antibody disease, granulomatosis with polyangiitis, and microscopic polyangiitis [1]. All etiologies arise from injury to the alveolar microcirculation with diffuse bleeding into the acinar portion of the lung [5].
Multiple case reports have reported post-operative DAH in the setting of inhaled anesthetic agent administration [6–8]. In our patient, NPPE was the likely triggering mechanism. This is supported by the observation that our patient was somnolent and had supraglottic airway obstruction needing jaw lift assistance in the PACU prior to the hemoptysis. Generation of a negative pleural pressure on inspiration against an upper airway obstruction is the mechanism of stress failure of the alveolar-capillary membrane in NPPE. Patients are typically young and male with no comorbidities as older patients may not be able to generate sufficient negative pressure for pulmonary capillary membrane rupture [3]. The role of NPPE in hemoptysis has previously been described with a suggested term of negative pressure pulmonary hemorrhage [4].
We believe that our patient had multiple contributing factors that predisposed him to diffuse alveolar hemorrhage. Cigarette smoke has a known injurious effect on alveolar epithelial cells due to oxidative stress from reactive oxygen species. This damage to the pulmonary interface through epithelial cell death is exacerbated by inhibition of normal repair processes [9]. Volatile anesthetics have also been reported to inhibit platelet function of varying degrees through largely varying mechanisms [10]. A study on the suppressive effects on platelet aggregation of volatile anesthetics suggested that halothane mediated its effects by reduction of the ligand-binding affinity of the platelet TXA2 receptor [11]. Sevoflurane also has been shown in vitro to have decreased platelet aggregation ratios in the intraoperative and early postoperative period [12]. Studies, however, detailing the clinical impact of potential antiplatelet properties are lacking.
We posit that sevoflurane has properties that, in patients predisposed to injury from pre-existing alveolar epithelial damage from smoking, may contribute to diffuse alveolar hemorrhage.
NPPE-related DAH is underdiagnosed but needs to be included in the differential of hemoptysis and acute respiratory failure after general anesthesia [3]. There have been three reported incidents of DAH in the setting of sevoflurane use [6,7,13]. All reported cases were in males with a mean age of 33 years presenting for elective surgical procedures. (Table 1)
Table 1.
Previous report cases of sevoflurane associated diffuse alveolar hemorrhage.
| S.N. | Characteristics | Kim et al | Khanna et al | Austin et al | Index case |
|---|---|---|---|---|---|
| Age (years) | 31 | 48 | 20 | 31 | |
| Sex | Male | Male | Male | Male | |
| Procedure | Excision of perirectal pilonidal cyst | Cataract removal with intraocular lens placement | Urethral stricture dilation via cystoscopy and retrograde urethrogram | Removal of foreign body from posterior humerus and debridement of fracture | |
| Treatment | IV methylprednisolone (1 g) daily for 3 days | Emergent reintubation; high PEEP; permissive hypercapnia | Supportive care | Supportive care | |
| Bronchoscopy | Hemorrhagic fluid in serial samples Cell count 92,000 red blood cells and 250 white blood cells (polymorphonuclear, 22%; lymphocytes, 15%; monocytes, 2%; macrophages, 56%) |
No active airway bleeding or obstructive mucous plugs | Diffuse erythema throughout tracheobronchial tree Sequential bronchoalveolar lavage showed progressively bloody return |
Deferred due to clinical improvement | |
| Other medications used | Midazolam Fentanyl |
Midazolam Propofol |
Propofol Fentanyl Midazolam |
Fentanyl Propofol |
|
| Substance abuse | Marijuana | Cocaine | None reported | None reported | |
| Post-operative Lab work | Hemoglobin 12.1 g/dL Platelet 246,000/μl Prothrombin time/international normalized ratio 12.9 seconds/1.0 Partial thromboplastin time (PTT) 23.3 |
Hemoglobin 9.4 g/dL White blood cell (WBC) 6.09 K/μl Platelet 210 K/μl INR 1.1 APTT 30.6 |
Hemoglobin 14.0 g/dL WBC 13.6 x 109/L Platelet count 157 x 109/L |
Hemoglobin 12.7 g/dL WBC 12.7 x 109/L Platelet count 226 x 109/L |
In a 48-year-old man, clinical deterioration on extubation prompted evaluation with CXR which showed bilateral upper and mid predominant airspace consolidation and air bronchograms. Lab work reported a drop in the hematocrit from baseline. An emergency bronchoscopy was negative for active airway bleeding or obstructive mucous plugs. A diagnosis of DAH was made and the patient was managed with high positive end-expiratory pressure (PEEP) and permissive hypercapnia. He improved over the next week and was successfully weaned off the ventilator [6].
In a 20-year old man, postoperative CXR was notable for patchy, peripheral consolidations bilaterally, sparing the apices and bases. A CT of the thorax showed patchy bilateral lung consolidation and ground glass opacities in an upper lobe distribution. A beside bronchoscopy showed diffuse tracheobronchial erythema with progressively bloody return from sequential bronchoalveolar lavage. A microbiological and serological workup was negative, and a diagnosis of DAH was made. The patient was managed conservatively and successfully extubated in three days. A follow-up CXR at one month had complete resolution of the infiltrates [13].
In a 31-year old man, postoperative hemoptysis, and hypoxemia with a PaO2 of 54 mm Hg 45 minutes after extubation prompted a CXR which revealed interval development of bilateral alveolar infiltrates. A CT chest angiography showed bilateral, centrally located ground glass opacities. Serum hemoglobin dropped and pulmonary function testing showed an elevated diffusing capacity. A bronchoscopy with bronchoalveolar lavage showed hemorrhage fluid in serial samples. Urinalysis was negative for RBCs and protein. A microbiological and serological workup was negative and a diagnosis of diffuse alveolar hemorrhage was made. The patient was managed with IV methylprednisolone (1 g) daily for three days with resolution of his hypoxemia. The patient was asymptomatic without recurrence one month after discharge [7].
We are currently unaware, to our knowledge, of any case reports of midazolam or fentanyl causing diffuse alveolar hemorrhage. Alveolar hemorrhage has been seen with medications including amiodarone and cytotoxic drugs [1,14].
DAH is a diagnostic challenge and failure to promptly identify and treat underlying etiologies may progress to acute respiratory failure [15]. The physician needs to be aware of the diagnosis so that early discontinuation or reversal of anti-platelet and anti-thrombotic medications may be performed [16]. In addition, supportive ventilation strategies may need to be employed including temporary intubation or positive end-expiratory pressure. Additional strategies to manage pulmonary edema including diuresis or fluid restriction may also need to be considered [4].
A range of in-hospital mortality has been reported from 20% to 100%, reinforcing need for early identification and treatment. Some predictors of in-patient mortality include shock, renal failure, and increased lactate dehydrogenase level [5]. Treatment targets the underlying diagnosis and options include corticosteroids, immunosuppressive agents, and occasionally plasmapheresis [1].
4. Conclusion
Sevoflurane is a colorless non-flammable volatile anesthetic that’s been used in clinical practice for twenty years [17]. Common respiratory reactions with sevoflurane include cough, laryngospasm, airway obstruction, and sialorrhea. Sevoflurane has been shown to selectively alter the ADP- and epinephrine-induced secondary aggregation of platelets thus contributing to a coagulopathy [10]. The relationship of sevoflurane and DAH has yet to be fully elucidated and multiple theories have been suggested. The authors of one case suggest that hemorrhage was from suspected coagulopathy and platelet dysfunction due to end-stage renal disease (ESRD) and cocaine abuse [6]. The authors of another case attribute hemorrhage to barotrauma from general anesthesia [8].
We believe that sevoflurane has properties that compounded existing alveolar epithelial damage from smoking, thus predisposing to hemoptysis from NPPE-related DAH.
NPPE-related DAH is an emergency that needs to be included in the differential of postoperative patients with hemoptysis. With preexisting alveolar damage from smoking and the administration of sevoflurane, negative intrapleural pressure generated from even transient upper airway obstruction may cause clinically significant alveolar capillary damage. Management strategies including temporary intubation or positive end-expiratory pressure and prompt discontinuation of anti-platelet and anti-thrombotic drugs may need to be considered.
Author Contribution
Contributors DH, SB, and SM contributed equally to writing the manuscript. DH and SB conducted the literature search. JK was primarily involved in patient care and was vital to the conception of the report. All the authors have given final approval for the version to be submitted.
Patient consent obtained.
Acknowledgments
Accepted for poster presentation at “South Central PA, Society of Hospital Medicine Annual Meeting”
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lara AR, Schwarz MI.. Diffuse Alveolar Hemorrhage. Chest. 2010;137(5):1164–1171. [DOI] [PubMed] [Google Scholar]
- [2].Larici AR, Franchi P, Occhipinti M, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol. 2014;20(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Contou D, Voiriot G, Djibré M, et al. Clinical features of patients with diffuse alveolar hemorrhage due to negative-pressure pulmonary edema. Lung. 2017;195(4):477–487. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz DR, Maroo A, Malhotra A, et al. Negative pressure pulmonary hemorrhage. Chest. 1999;115(4):1194–1197. [DOI] [PubMed] [Google Scholar]
- [5].de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: factors associated with in-hospital and long-term mortality. Eur Respir J. 2010;35(6):1303–1311. [DOI] [PubMed] [Google Scholar]
- [6].Khanna AK, Cummings KC. Pulmonary hemorrhage in an outpatient ophthalmic anesthesia setting - it’s never “just a cataract. J Anaesthesiol Clin Pharmacol. 2012;28(4):520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim CA, Liu R, Hsia DW. Diffuse alveolar hemorrhage induced by sevoflurane. Ann Am Thorac Soc. 2014;11(5):853–855. [DOI] [PubMed] [Google Scholar]
- [8].Kim JP, Park JJ, Kim NJ, et al. A case of diffuse alveolar hemorrhage after tonsillectomy -A case report-. Korean J Anesthesiol. 2012;63(2):165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1(3):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hirakata H, Nakamura K, Sai S, et al. Platelet aggregation is impaired during anaesthesia with sevoflurane but not with isoflurane. Can J Anaesth. 1997;44(11):1157–1161. [DOI] [PubMed] [Google Scholar]
- [11].Hirakata H, Ushikubi F, Narumiya S, et al. The effect of inhaled anesthetics on the platelet aggregation and the ligand-binding affinity of the platelet thromboxane A2 receptor. Anesth Analg. 1995;81(1):114–118. [DOI] [PubMed] [Google Scholar]
- [12].Doğan IV, Ovali E, Eti Z, et al. The in vitro effects of isoflurane, sevoflurane, and propofol on platelet aggregation. Anesth Analg. 1999;88(2):432–436. [DOI] [PubMed] [Google Scholar]
- [13].Austin A, Modi A, Judson MA, et al. Sevoflurane induced diffuse alveolar hemorrhage in a young patient. Respir Med Case Rep. 2017;20:14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iskandar SB, Abi-Saleh B, Keith RL, et al. Amiodarone-induced alveolar hemorrhage. South Med J. 2006;99(4):383–387. [DOI] [PubMed] [Google Scholar]
- [15].Scollo V, Zanoli L, Russo E, et al. A case of rare diffuse alveolar hemorrhage and review of literature. Clin Med Insights Case Rep. 2017;10:1179547617726077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choe YS, Kim NY, Lee AR. Diffuse alveolar hemorrhage in patients undergoing neurointervention: a case report. Anesth Pain Med. 2016;6(5):e33979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De Hert S, Moerman A. Sevoflurane. F1000Res. 2015August;4:626. [DOI] [PMC free article] [PubMed] [Google Scholar]


