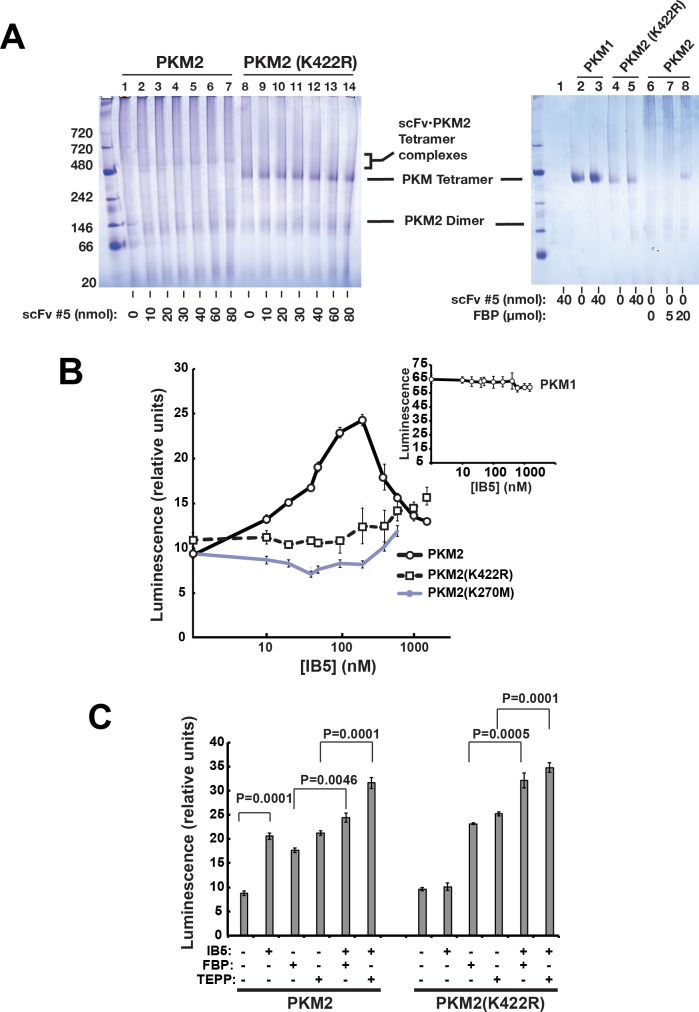
Fig 4. Recombinant scFv 5 induced tetramerization of recombinant PKM2 and stimulated its glycolytic activity in vitro.
A. Blue native gel electrophoresis. This method resolved the dimer and tetramer forms of PKM2. Left: A monovalent form of scFv 5, produced in E. coli, induced tetramer formation in WT PKM2 along with a bandshift whose magnitude was dependent on the input amount of scFv 5. The mutant PKM2 (K422R) was constitutively tetrameric and did not exhibit a bandshift in the presence of scFv 5. Reaction volume was 20 μl. Right: scFv 5 did not produce a band shift with recombinant PKM1, which ran as a tetramer; added FBP produced the tetramer form of WT PKM2 (lanes 6–8). B. scFv 5 stimulated glycolytic activity of WT and mutant PKM2. Activity was measured by Kinase-Glo Plus Luminescent Kinase Assay kit (Promega), using ADP and PEP as substrates, with PKM2 at 50 nM. Shown are values with the basal activity of PKM2 alone subtracted out. Inset: scFv 5 did not affect PKM1’s glycolytic activity. C. IB5 stimulated the glycolytic activity of K422R mutant PKM2 to a greater extent in the presence of allosteric activators FBP or TEPP-46. Note: underlying data are included in corresponding tabs in the accompanying supplemental Excel file S1 Data. FBP, fructose-1,6-bisphosphate; IB5, intrabody 5; PEP, phosphoenolpyruvate; PKM2, pyruvate kinase isoform M2; scFv, single-chain variable fragment; WT, wild-type.