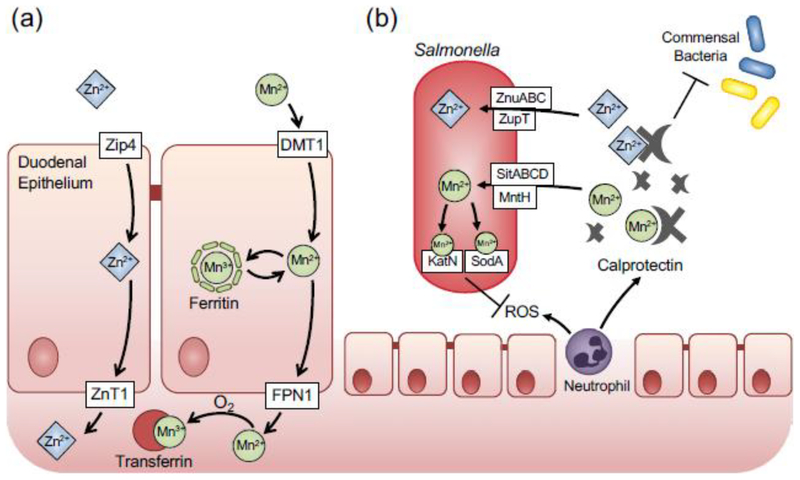
Fig. 2. Manganese and zinc homeostasis and subversion of nutritional immunity by Salmonella Typhimurium.
(a) Manganese and zinc uptake by the mammalian host in the duodemum. Zinc is imported into epithelial cells by Zrt- and Irt-like protein 4 (Zip4), and released on the basolateral side by Zinc Transporter 1 (ZnT1). Akin to iron, manganese is transported across the apical membrane of duodenal epithelial cells by the Divalent Metal Transporter 1 (DMT1). stored in ferritin. or exported on the basolateral side via ferroportin (FPN1). Free manganese is sequestered by transferring and transported to target organs, (b) During intestinal inflammation, zinc and manganese are sequestered by calprotectin. which is released by epithelial cells and neutrophils. In addition, zinc-binding protein metallothionein is expressed in the colonic mucosa in the inflamed intestine. Calprotectin-mediated metal withholding suppresses growth of commensal bacteria Enteric pathogen Salmonella uses several high-affinity transporters to subvert nutritional immunity and sidestep the competition with the commensal bacteria. Zinc-dependent superoxide dismutases and catalases help Salmonella defend against reactive oxygen species (ROS).