Figure 1.
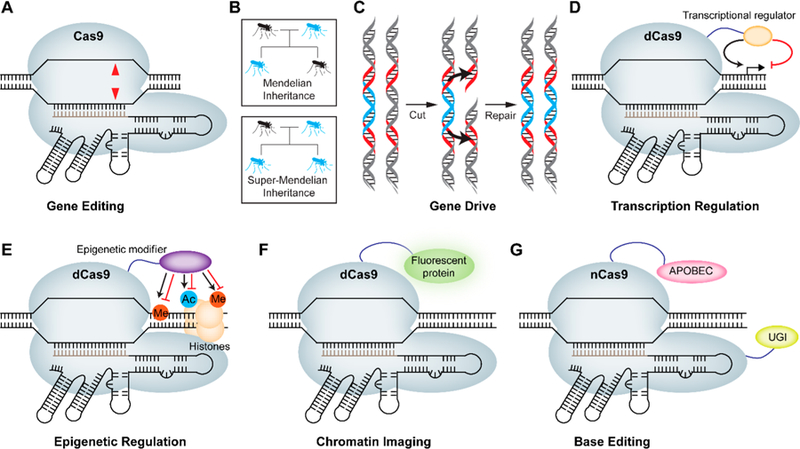
Major applications of CRISPR-Cas9. (A) Cas9 enables gene editing through its RNA-guided DNA endonuclease activity leading to double-stranded breaks (red triangles). (B) CRISPR-Cas9-based gene drive allows self-propagation of an engineered gene and/or trait in a species population. The probability of passing on an engineered gene (blue) to the progeny in Mendelian inheritance is 50%, while the same probability for gene drives approaches 100%. (C) The molecular mechanism of super-Mendelian inheritance in the gene drive involves a Cas9-induced double-stranded break on the wild type allele (gray) that is repaired by copying the drive allele (blue) from the engineered parent via HDR, causing replacement of the wild type allele with the drive element. Red regions indicate homology arms. (D) Nuclease dead Cas9 (dCas9) can be fused to an activator or repressor domain to regulate gene expression. (E) dCas9 can be fused to DNA-demethylating enzymes/methyltransferase as well as histone demethylase/methyltransferase or deacetylase/acetyltransferase domains to regulate epigenetic modifications in a sequence-specific manner. (F) dCas9 can be fused to a fluorescent protein such as GFP to enable imaging of a specific locus. (G) Nickase Cas9 (nCas9) can be fused to cytosine deaminase (APOBEC1) and uracil glycosylase inhibitor (UGI) to enable conversion of cytosine (C) to thymine (T) without double-stranded breaks.
