Abstract
Purpose
Endometrioid endometrial cancer is strongly associated with obesity and insulin resistance. Metformin,an insulin sensitizer, reduces endometrial tumor growth in vitro. Presurgical window studies allow rapid in vivo assessment of antitumor activity. Previous window studies found metformin reduced endometrial cancer proliferation but these lacked methodological rigor. PREMIUM measured the anti-proliferative effect of metformin in vivo using a robust window study design.
Patients and Methods
A multicenter, double-blind, placebo-controlled trial randomized women with atypical hyperplasia or endometrioid endometrial cancer to receive metformin (850 mg daily for 3 days, and twice daily thereafter) or placebo for 1 to 5 weeks until surgery. The primary outcome was posttreatment IHC expression of Ki-67. Secondary outcomes investigated the effect of metformin on markers of the PI3K–Akt–mTOR and insulin signaling pathways and obesity.
Results
Eighty-eight women received metformin (n = 45) or placebo (n = 43) and completed treatment. There was no overall difference in posttreatment Ki-67 between the metformin and placebo arms, in an ANCOVA analysis adjusting for baseline Ki-67 expression (mean difference −0.57%; 95% CI, −7.57%–6.42%; P = 0.87). Metformin did not affect expression of markers of the PI3K–Akt–mTOR or insulin signaling pathways, and did not result in weight loss.
Conclusions
Short-term treatment with standard diabetic doses of metformin does not reduce tumor proliferation in women with endometrioid endometrial cancer awaiting hysterectomy. This study does not support a biological effect of metformin in endometrial cancer and casts doubt on its potential application in the primary and adjuvant treatment settings.
Introduction
The rising global incidence of endometrioid endometrial cancer and the associated increase in deaths from the disease represents a significant health challenge (1, 2). New strategies for both the prevention and treatment of endometrioid endometrial cancer are required, especially for women who wish to preserve fertility and those for whom obesity and associated co-morbidities mean hysterectomy is a hazardous procedure. The standard management of advanced and recurrent disease is currently limited to chemo- and radiotherapy, with poor response rates (3). Targeted multi-modal treatments may offer hope and a safe oral maintenance medication that prevents recurrence could have a major impact.
Endometrioid endometrial cancer is driven by obesity and insulin resistance (4). Metformin improves insulin sensitivity and epidemiological evidence has suggested that its use is associated with improved survival in women with endometrial cancer (5). In vitro, it reduces endometrioid endometrial cancer cell proliferation, invasion and migration and increases apoptosis (6–8). Metformin inhibits complex I of the respiratory electron transport chain in hepatocyte mitochondria, preventing gluconeogenesis and lowering serum glucose. It indirectly lowers levels of insulin, itself a potent stimulator of endometrial cancer growth (9) and downregulates expression of insulin and related growth factors receptors (10–12), inhibits phosphorylation and activation of the insulin-like growth factor 1 receptor (10), and increases expression of insulin-like growth factor binding protein-1 (12). Furthermore, metformin may act directly on endometrial cancer cells, inhibiting signaling through key oncogenic pathways, including the PI3K–Akt–mTOR pathway, via AMPK, the MAPK/ERK transcriptional control pathway as well as those controlled by insulin and related growth factor receptors (9). The relevance of these actions for the anti-proliferative effects in endometrioid endometrial cancer remains unknown.
The presurgical window study design is both a time- and cost-efficient means of determining the effect of safe drugs on untreated tumors in vivo. To date, six window studies of metformin in endometrioid endometrial cancer have been performed (13–18). All have been small, non-randomized, open-label trials, and, with the exception of two studies, including our own phase 2 feasibility study, failed to include contemporaneous controls for comparison (17, 18). Five of the six studies found a reduction in endometrial cancer proliferation, as measured by IHC expression of Ki-67, with metformin treatment (13–15, 17, 18). The exception was the study by Schuler and colleagues (16), although their median treatment window of 10 days may have been too short for any biological effect.
Previous studies observed a reduction in apoptosis and signaling through the PI3K–Akt–mTOR and MAPK/ERK pathways with metformin exposure (13, 14, 16, 18), although this was not consistent across all studies (15, 17). Indeed, in our own trial, there was a reduction in downstream markers of the PI3K–Akt–mTOR pathway (pS6, pAKT, p4EBP1) in both metformin-treated and untreated groups and we proposed that this may be related to differences in tumor sampling before and after intervention (17). At baseline, the cancer was sampled in situ using an outpatient sampling device, but at hysterectomy, tumor was sampled from the resected uterus by the pathologist. The latter is subject to hypoxia during surgical resection and to delays in formalin penetration and fixation, which are likely to influence expression of unstable phosphorylated biomarkers (19). Expression of Ki-67, a more stable protein, is less likely to be affected by sampling methodology.
Although earlier studies suggested a potential benefit from metformin treatment in reducing tumor growth in endometrioid endometrial cancer, the quality of evidence was low because of inadequate methodology. An adequately powered, placebo-controlled, double-blind, randomized trial was therefore required to address the limitations of previous work to provide conclusive evidence of an antiproliferative effect of metformin in endometrioid endometrioid endometrial cancer. Such evidence would provide support for large-scale international clinical efficacy studies of its use in the primary treatment or adjuvant settings.
Patients and Methods
Trial design and participants
A phase III, double-blind, placebo-controlled, randomized presurgical window trial (PREMIUM) was conducted in five hospitals within the North West of England.
Eligible women were aged 18 years or older, had biopsy proven atypical endometrial hyperplasia (AEH; ref. 20) or endometrioid endometrial cancer and were scheduled to undergo surgical treatment by hysterectomy in the following 5–35 days. AEH is considered a precursor of endometrioid endometrial cancer due to its similar mutational profile and rate of progression to cancer of up to 40% within 12 months (21). Treatment is thus primarily surgical. Exclusion criteria included nonendometrioid endometrial cancer, a window period shorter than 5 days, nonsurgical treatment planned, pregnancy, current metformin or hypoglycemic medication use, severe renal (serum creatinine >130 μmol/L or eGFR <45 mL/m/1.732 m2) or hepatic impairment (results discussed on an individual basis with a hepatologist), allergy to biguanides, current alcohol abuse (>14 U/week), progesterone, mTOR inhibitor or other chemotherapeutic drug use and inability to give informed consent.
Participants were identified through two multidisciplinary meetings held at the tertiary referral units on a weekly basis. At the recruitment visit, a medical history was taken, serum renal and liver function determined and a pregnancy test (if applicable) performed. All participants provided written, informed consent. The trial was sponsored by Manchester University NHS Foundation Trust and was approved by the North West Research Ethics Committee (14/NW/1236), Medicine and Healthcare Products Regulatory Authority (MHRA, reference 21387/0232/001-0001) and local Research and Development departments. The trial was prospectively registered on the European (EudraCT number 2014-000991-25) and UK (ISRCTN 88589234) clinical trial databases and conducted in accordance with Good Clinical Practice guidelines.
Randomization and masking
Participants were randomized to either metformin (850 mg, Medley Pharma Ltd) or placebo, to be taken after food once daily for 3 days increased to twice daily thereafter until the evening before surgery, the date of which was determined by local surgeon availability (1–5 weeks). Computer generated randomization lists were produced in advance of trial commencement by an independent clinical trials unit (Manchester Academic Health Science Centre-Trials Co-ordination Centre, MAHSC-CTU) using the permuted block method, creating four blocks of size 30 (1:1 metformin: placebo). The lists were used during drug packaging into tamper-proof bottles containing 70 tablets by the manufacturer (Pharmacy Manufacturing Unit, Guys and St Thomas' NHS Foundation Trust), ensuring each bottle was labeled with a unique two letter code. Following recruitment, participants were randomized by computer to the next available drug bottle, identified by its unique code, by telephoning the central randomization line at MAHSC-CTU, with bottles dispensed from the Clinical Trials Pharmacy at Manchester University NHS Foundation Trust (MFT).
Participants, investigators, histopathologists, and the clinical trial team at MAHSC, with the exception of the trial statistician, remained blind to treatment allocation for the duration of trial recruitment, participation and analysis. This was achieved by using a placebo and active drug that were identical in appearance, packaging, labeling and instructions for use. In the event of a serious adverse event, where urgent knowledge of treatment allocation was required to ensure appropriate patient care, emergency unblinding was possible by contacting the on call pharmacist at MFT, who had access to sealed, opaque envelopes containing details of treatment assignment. This was not required at any point in the trial.
Procedures
At recruitment, anthropometric measurements were performed, including height, weight, and body mass index (BMI; full description in Supplementary Methods).
Serum obtained by venipuncture following a 6-hour fast was used to quantify insulin sensitivity by measuring glucose, insulin and glycated hemoglobin (HbA1C) and also to measure adiponectin, leptin, IGF-1 and IGFBP-1 levels. Analyses were conducted by automated assay in the clinical biochemistry department at MFT using standardized operating procedures, with the exception of adiponectin, leptin, IGF-1 and IGFBP-1, which were measured using an ELISA kit (Quantikine [IGF-1] and DuoSet [adiponectin, leptin, IGFBP-1] ELISA Kits, R&D Systems).
The endometrial biopsy taken for clinical diagnosis was requested from the local hospital in the form of a formalin-fixed, paraffin-embedded block (FFPE).
Participants kept a contemporaneous study diary to document treatment compliance and adverse events, which was supported by at least weekly telephone contact with the research team. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was used to grade events (22). In the case of intolerable side effects, dose modifications were permitted as advised by a principal or coinvestigator.
On the morning of surgery (final visit), unused study drug was returned and the number of tablets counted and compared with the study diary. Repeat anthropometric measurements were performed and fasted serum obtained for the aforementioned analyses. Trough metformin levels were measured in plasma 12 hours after the last dose of study medication by liquid chromatography and tandem mass spectrometry. Analytical separation was performed by hydrophilic interaction chromatography as described by Nielsen and colleagues (23). Quantification was performed in the Department of Clinical Pharmacology and Pharmacy, University of Southern Denmark.
A repeat endometrial biopsy was performed under general anesthetic using an endometrial sampling device immediately prior to hysterectomy. The sample was placed directly into formalin for fixation and later embedded in paraffin. An FFPE block was also obtained from the hysterectomy specimen for immunohistochemical analysis. If hysterectomy was delayed beyond five weeks from commencement of the study drug, participants attended an outpatient clinic for repeat endometrial sampling, and the resultant FFPE block was used for all study analyses.
Changes made to the trial design during participant recruitment and analysis are detailed in supplementary methods.
Outcomes
The primary outcome was posttreatment Ki-67 expression in the hysterectomy specimen, adjusted for baseline Ki-67 expression. Automated IHC staining was performed centrally using the Leica Bond Max (Leica Biosystems) and heat-induced epitope retrieval on 4-μm whole sections cut from FFPE blocks from the diagnostic endometrial biopsy and hysterectomy specimen (Supplementary Table 1). Primary antibody detection was performed using the Refine Detection Kit (Leica Biosystems), containing a rabbit anti-mouse IgG secondary antibody and anti-rabbit poly-HRP IgG antibody and uses 3,3′-diaminobenzidine as a chromogen. Slides were counterstained with hematoxylin. Positive and negative (isotype) controls were employed for quality assurance.
Whole slides were digitized using the Leica SCN400 Slide Scanner (Leica Microsystems) and were scored using the hotspot method and semi-automated Definiens Developer software, as described previously (24). In brief, three areas of greatest endometrial cancer proliferation (hotspots) were manually selected at ×10 magnification and the number of positively stained nuclei recorded as a percentage of the total number of nuclei scored using an optimized solution.
Secondary outcomes included tolerability of treatment (treatment compliance and adverse events), the effect of metformin on anthropometric and serum markers of obesity and insulin resistance (fasting serum glucose, insulin, HbA1C, adiponectin, leptin, IGF-1 and IGFBP-1), intratumoral insulin signaling (pIR, total and phosphorylated IGF1R, IGFBP1), the PI3K–Akt–mTOR pathway (pACC, pAkt, pS6, p4EBP1) and apoptosis (cleaved caspase-3), as determined by IHC.
Secondary endpoints were assessed using tissue microarrays, constructed from triplicate cores obtained from representative areas of pre- and postintervention endometrial biopsies, pre-selected by a specialist gynecologic consultant histopathologist. Full details of the antibodies and experimental conditions used for the secondary outcome IHC markers are provided in Supplementary Table 1. Primary antibody incubation was for one hour in all cases. Using Definiens Developer, the entire core was scored and either the percentage of positively stained cells, regardless of stain intensity (cleaved caspase-3), or an H-score, the product of stain intensity (0 = none, 1 = mild, 2 = moderate, 3 = strong) and percentage area stained (0%–100%) recorded.
All scoring was performed by two independent observers, with disagreements settled by consensus, and the mean score used in the analysis.
Statistical analysis
On the basis of our pilot study results (17), we estimated that 88 women would be required to detect a 10% difference in posttreatment Ki-67 expression in an intention-to-treat analysis with 90% power at the 0.05 level, assuming the SD in the control and intervention arms is 28.6% and 20.6%, respectively, and the correlation between baseline and post-treatment Ki-67 expression is 0.82.
Ninety-three women were recruited to allow for the replacement of participants who withdrew from the study before taking any trial medication and those who discontinued the treatment >72 hours prior to surgery, as this may lead to a rebound increase in endometrial cancer proliferation (20). A modified intention-to-treat analysis was therefore performed including all participants who had taken at least one dose of the drug and for whom a tumor biopsy was available within 72 hours of treatment discontinuation.
Descriptive statistics were summarized using the mean and SD or median and interquartile range. A linear model (ANCOVA), adjusting for baseline expression, was used to estimate the effect of metformin on primary and secondary outcomes. Exploratory, a priori, subgroup analyses investigated whether baseline BMI, insulin resistance (measured by either homeostatic model assessment-insulin resistance [HOMA-IR] or HbA1C), grade of endometrial cancer and total dose of metformin were trial arm effect modifiers of posttreatment Ki-67 expression.
Compliance and adverse event reporting are described in Supplementary Methods.
The statistical analysis was performed using Stata version 13. A P value ≤0.05 was considered statistically significant.
Results
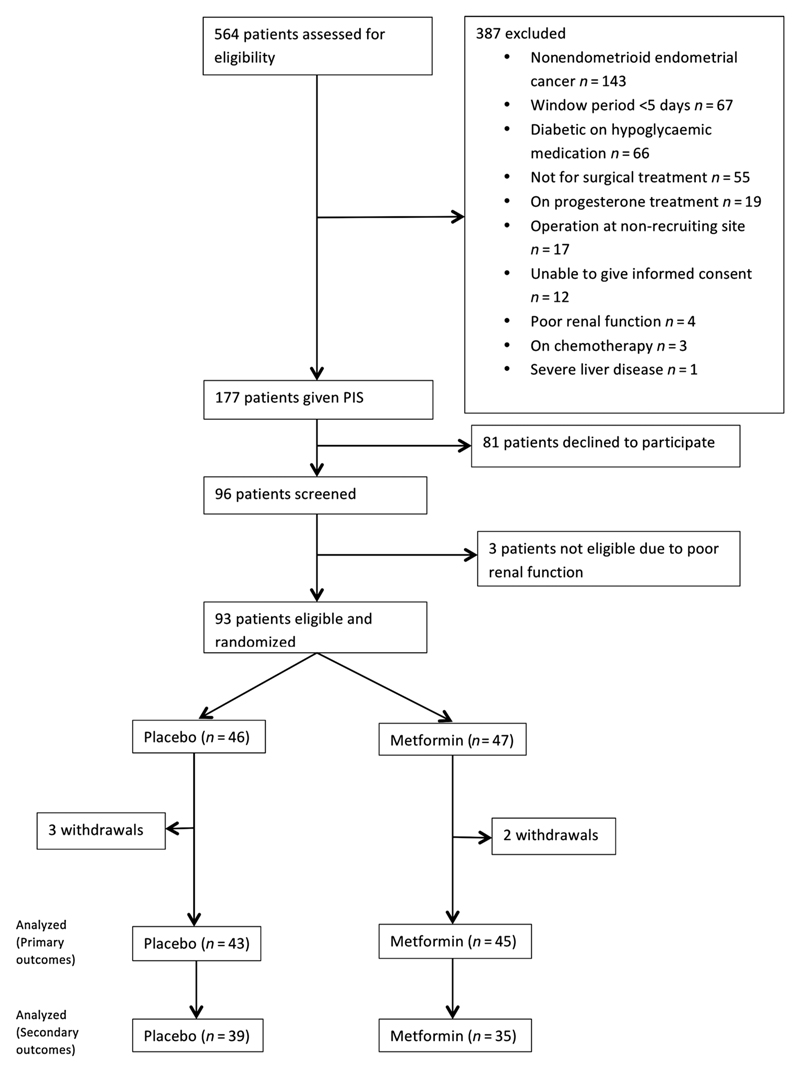
Between February 2015 and 2017, 564 women were assessed for eligibility, of whom 387 met at least one exclusion criteria (Fig. 1). Of the 177 women approached, 96 (54.2%) agreed to participate and 93 had their eligibility confirmed and were randomized. There were five withdrawals (metformin = 2, placebo = 3), of which only one commenced the trial medication. In this instance, the date of surgery was prolonged beyond five weeks from randomization and a repeat endometrial biopsy was not possible due to cervical stenosis, meaning no tumor was available for the final analysis. Eighty-eight participants completed treatment and were included in the modified intention-to-treat analysis. Paired representative pre- and posttreatment endometrial biopsies of sufficient size to perform IHC for secondary outcomes were available for 39 women in the placebo arm and 35 in the metformin arm (Fig. 1).
Figure 1.
PREMIUM CONSORT diagram.
The mean duration of treatment in the metformin arm was 20.5 ± 7.3 days and 21.5 ± 6.6 days in the placebo arm. Demographic and baseline characteristic data were balanced across the treatment groups (Table 1). Premenopausal women comprised 18.1% of participants. As the majority of these had irregular bleeding, it was not possible to determine the timing of the biopsy in relation to the menstrual cycle. Over half of participants were obese, with almost one fifth having a BMI ≥ 40kg/m2. The median waist:hip ratio in both groups was close to 0.85, the threshold used by the World Health Organisation to define abdominal adiposity (26).
Table 1. Demographic and baseline characteristic data.
| Characteristics | Metformin (n = 45) | Placebo (n = 43) |
|---|---|---|
| Age, years | 64.0 (29.6–83.7) | 67.1 (39.8–85.2) |
| Premenopausal | 9 (20.0) | 7 (16.3) |
| Postmenopausal | 36 (80.0) | 36 (83.7) |
| BMI, kg/m2 | 31.0 (20.2–54.2) | 32.0 (17.8–47.6) |
| <25 | 8 (17.8) | 9 (20.9) |
| 25–29.9 | 11 (24.4) | 10 (23.3) |
| 30–34.9 | 11 (24.4) | 11 (25.6) |
| 35–39.9 | 8 (17.8) | 5 (11.6) |
| ≥40 | 7 (15.6) | 8 (18.6) |
| Waist:hip ratio | 0.84 (0.74–0.99) | 0.83 (0.70–0.96) |
| Diagnosed | ||
| Diabetes | 0 (0.0) | 2 (4.7) |
| Non-diabetic hyperglycemia | 3 (6.7) | 1 (2.3) |
| Undiagnosed | ||
| Diabetes | 1 (2.2) | 0 (0.0) |
| Non-diabetic hyperglycemia | 7 (15.6) | 3 (7.0) |
| Tumour grade at hysterectomy | ||
| AEH | 2 (4.4) | 2 (4.7) |
| 1 | 26 (57.8) | 23 (53.5) |
| 2 | 10 (22.2) | 12 (27.9) |
| 3 | 6 (13.3) | 6 (14.0) |
| 4a | 1 (2.2) | 0 (0.0) |
| FIGO stage at hysterectomy | ||
| 1a | 29 (64.4) | 23 (53.5) |
| 1b | 4 (8.9) | 11 (25.6) |
| 2 | 1 (2.2) | 4 (9.3) |
| 3 | 9 (20.0) | 3 (7.0) |
| 4 | 0 (0.0) | 0 (0.0) |
| N/A | 2 (4.4) | 2 (4.7) |
| ER expression | ||
| Positive | 44 (88.9) | 43 (100.0) |
| Negative | 1 (2.2) | 0 (0.0) |
| PR expression | ||
| Positive | 42 (93.3) | 42 (97.7) |
| Negative | 3 (6.7) | 1 (2.3) |
| p53 status | ||
| Wild type | 39 (86.7) | 40 (93.0) |
| Mutant/Null | 4 (8.9) | 2 (4.7) |
| Missing cores | 2 (4.4) | 1 (2.3) |
NOTE: Data are median (range) or n (%). Glycemic status was determined according to clinical history or baseline HbA1C, with nondiabetic hyperglycemic and type II diabetes defined as an HbA1C >42 mmol/mol and >48 mmol/mol, respectively.
Abbreviations: AEH, atypical endometrial hyperplasia; ER, estrogen receptor; PR, progesterone receptor.
Grade 4 is used to refer to an undifferentiated endometrial cancer, which may occur in pure form, with evidence of epithelial differentiation in only a few tumor cells, or in combination with a low grade endometrioid adenocarcinoma (1).
1. Royal College of Pathologists. Standards and datasets for reporting cancers Dataset for histological reporting of endometrial cancer. 2017
Diabetes and nondiabetic hyperglycemia had been previously diagnosed in 7% of women, and a further 22% of participants had previously unrecognized nondiabetic hyperglycemia, consistent with previous findings (22). One woman in the metformin arm was diagnosed with type II diabetes on her baseline HbA1C measurement.
As anticipated, >85% of women had disease confined to the uterus (stage I and II) at the time of surgery. The hormonal receptor and mutational status of tumors was relatively homogeneous, with almost all estrogen and progesterone receptor positive. Fewer than 10% of tumors showed mutant/null p53 expression.
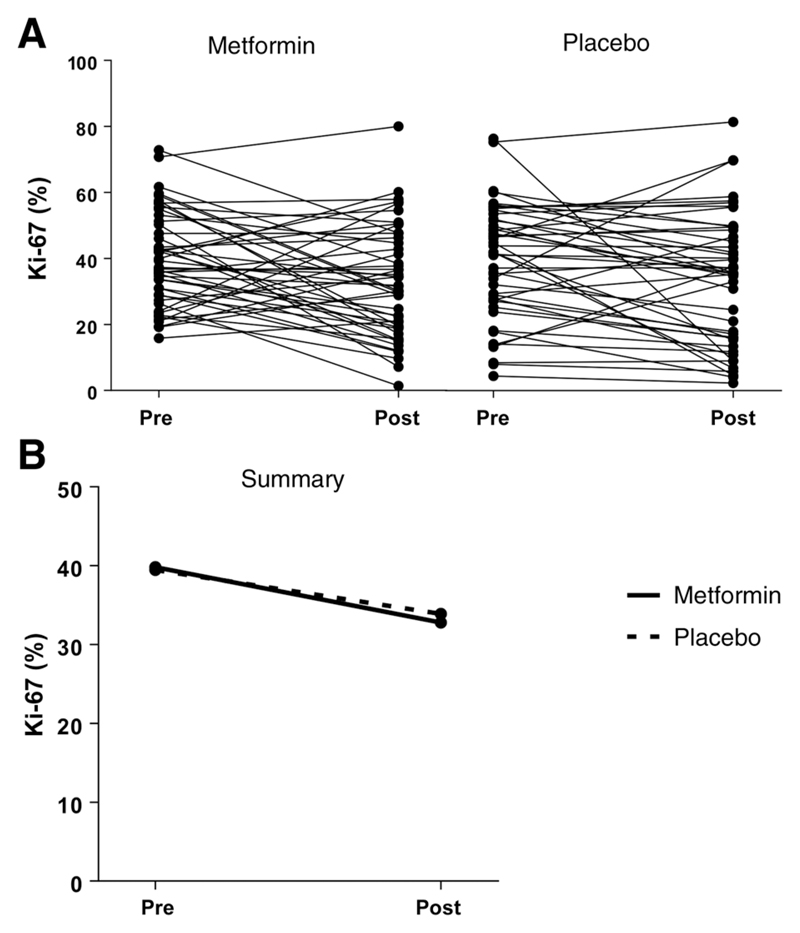
Overall, metformin treatment had no effect on Ki-67 expression (Fig. 2). The mean difference between the metformin and placebo arms in posttreatment Ki-67 expression after adjustment for baseline expression was −0.57% [95% confidence interval (CI), −7.57%–6.42%; P = 0.87].
Figure 2.
Effect of metformin treatment on Ki-67 expression. A, Pre- and posttreatment Ki-67 expression in endometrial cancers from individual participants. B, Mean Ki-67 expression pre- and posttreatment with metformin or placebo. There was no significant difference in overall posttreatment Ki-67 expression between the metformin and placebo arms after adjusting for baseline Ki-67 expression. In the metformin arm, mean Ki-67 expression decreased from 39.8% (35.7%–43.9%) at baseline to 32.8% (95% CI, 28.0%–37.6%) following treatment, whereas in the placebo arm, mean Ki-67 expression decreased from 39.5% (95% CI, 34.5%–44.5%) to 33.9% (95% CI, 28.2%–39.7%). The mean difference in posttreatment Ki-67 expression between the metformin and placebo arms was −0.57% (95% CI, −7.57%–6.42%; P = 0.87), after adjusting for baseline Ki-67 expression.
In an exploratory subgroup analysis, baseline BMI was a significant metformin treatment effect modifier when a BMI cutoff of 30 kg/m2 was used (P = 0.05). Participants with a BMI <30 kg/m2 had a nonsignificant decrease in posttreatment Ki-67 expression of −8.31% (95% CI, −18.70–2.09) with metformin treatment, whereas participants with a BMI ≥30 kg/m2 had a nonsignificant increase of +5.50% (95% CI, −3.57–14.57) in posttreatment Ki-67 expression in the metformin arm (Supplementary Fig. S1). This finding was not confirmed at other BMI cutoffs or when BMI was handled as a continuous variable, there was no evidence for a dose–response relationship between BMI and metformin treatment effect and neither was there any association between BMI and change in serum glucose, metformin plasma levels, or any other secondary endpoint. There was no significant difference in response to metformin treatment in any of the other subgroups (insulin resistance, tumor grade, total dose of metformin received) analyzed (all P > 0.05).
Metformin use was not associated with a significant increase in AMPK activity, as measured by expression of its substrate pACC (mean difference 11.18; 95% CI, −10.59–32.96, P = 0.31). Neither was there a reduction in signaling through the PI3K–Akt–mTOR pathway, detected as either a reduction in pS6 (mean difference −15.96; 95% CI, −37.60–5.69; P = 0.15), p4EBP1 (mean difference 2.16; 95% CI, −17.19–21.51; P = 0.82), or pAKT (mean difference −5.08; 95% CI, −20.39–10.24; P = 0.51) expression with metformin exposure, compared with the placebo group.
As expected, posttreatment serum glucose levels were significantly lower in women in the metformin group compared with those in the placebo arm (mean difference −0.40 mmol/L, 95% CI, −0.68 to −0.11; P = 0.007; Table 2). This did not translate into improvements in insulin sensitivity or to a reduction in signaling through the insulin and IGF1R receptors within the endometrial tumors (Table 2).
Table 2. Effect of metformin on serum markers of insulin resistance and intratumoral insulin signaling.
| Serum and tumor markers | Mean difference (95% CI) | P |
|---|---|---|
| Serum | ||
| Glucose (mmol/l) | −0.40 (−0.68 to −0.11) | 0.007** |
| Insulin (log pmol/l) | −0.07 (−0.31–0.17) | 0.57 |
| HOMA-IR (log) | −0.16 (−0.43–0.12) | 0.26 |
| HbA1C (mmol/mol) | −0.53 (−1.31–0.25) | 0.18 |
| IGF-1 (ng/mL) | 2.05 (−4.90–8.99) | 0.56 |
| IGFBP1 (log ng/mL) | −0.18 (−0.49–0.13) | 0.25 |
| Adiponectin (mg/l) | −0.15 (−0.35–0.05) | 0.13 |
| Tumor | ||
| pIR | −1.66 (−23.03–19.71) | 0.88 |
| pIGF1R | 6.67 (−10.92–24.26) | 0.45 |
| IGFBP1 | 6.08 (−26.85–39.01) | 0.71 |
NOTE: The mean difference (metformin-placebo) in posttreatment marker level/expression is shown, adjusting for baseline marker level/expression. HOMA-IR calculated as (serum glucose × insulin)/22.5. **, P ≤ 0.01.
Metformin treatment was not associated with a significant increase in apoptosis. The mean difference in log cleaved caspase-3 expression between the metformin and placebo arms was −0.14 (95% CI, −0.80–0.53; P = 0.69).
The mean difference in posttreatment weight was not statistically significant (mean difference −0.76 kg; 95% CI, −1.54–0.03; P = 0.06). Similarly, exposure to metformin had no effect on the waist:hip ratio (mean difference −0.004; 95% CI, −0.01–0.02; P = 0.60) or serum leptin levels (mean difference, −3.54 ng/mL; 95% CI, −8.83–1.75; P = 0.19).
Given the varying interindividual responses to metformin, change in Ki-67 expression was measured against changes in secondary outcome measures. No significant correlations were found (all P > 0.05; Table 3).
Table 3. Changes in Ki-67 expression with metformin treatment did not correlate with changes in expression of markers of the PI3K–Akt–mTOR or insulin signaling pathways within endometrial tumors.
| Difference in | pAkt expression | pS6 expression | p4EBP1 expression | pACC expression | pIR expression | pIGF1R expression | Serum insulin | HOMA-IR |
|---|---|---|---|---|---|---|---|---|
| Ki-67 expression | −0.18, 0.32 | −0.11, 0.54 | 0.19, 0.27 | −0.08, 0.63 | −0.19, 0.27 | 0.17, 0.32 | 0.22, 0.15 | 0.22, 0.14 |
NOTE: Results are presented as Pearson correlation coefficient, P value.
Compliance with the trial medication was good, with 80.0% (36/45) and 86.0% (37/43) in the metformin and placebo arms missing two or fewer doses, respectively. This was confirmed when trough metformin levels were measured; all participants randomized to receive active drug had detectable levels of metformin in their plasma. No correlation was found between trough metformin levels and change in Ki-67 expression, however (r = 0.08; 95% CI, −0.22 to −0.38; P = 0.59). Metformin was generally well tolerated. Women in the metformin arm reported more adverse events, which were of greater severity than those experienced by women in the placebo arm (P = 0.001), but they did not discontinue treatment (Table 4). These were predominately gastrointestinal, including more nausea and vomiting, diarrhea, and anorexia.
Table 4. Adverse events.
| Adverse events | Metformin, n (%) | Placebo, n (%) | P |
|---|---|---|---|
| Worst grade | 0.001*** | ||
| Grade 0 | 8 (17.8) | 19 (43.2) | |
| Grade 1 | 26 (57.8) | 23 (52.3) | |
| Grade 2 | 10 (22.2) | 2 (4.7) | |
| Grade 3 | 1 (2.2) | 0 (0.0) | |
| Abdominal pain | 11 (24.4) | 6 (13.6) | 0.283 |
| Nausea and vomiting | 17 (37.8) | 6 (13.6) | 0.015* |
| Diarrhea | 23 (51.1) | 6 (13.6) | 0.0003*** |
| Flatulence/bloating | 6 (13.3) | 2 (4.5) | 0.267 |
| Anorexia | 9 (20.0) | 0 (0.0) | 0.003** |
| Taste disturbance | 3 (6.7) | 2 (4.5) | 1.000 |
| Rash/itching | 6 (13.3) | 2 (4.5) | 0.267 |
| Renal dysfunction | 1 (2.2) | 0 (0.0) | 1.000 |
NOTE: There were 28 unique preferred terms each recorded in at least one participant. The worst grade within an individual across all 28 terms was reported and compared using the Wilcoxon signed rank test. Eight of the 28 terms most likely to be related to metformin exposure are shown here. For these entries, the numbers of cases experiencing any grade (1–3) of a specific adverse event were recorded and compared using Fisher exact tests. For the purpose of adverse event reporting, all participants taking at least one dose of the trial medication were included (metformin n = 45, placebo n = 44). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Discussion
We report the first randomized controlled trial of pre-hysterectomy metformin in endometrial cancer, which found no reduction of tumor proliferation with short-term treatment. Metformin did not affect apoptosis or the PI3K–Akt–mTOR pathway in endometrioid endometrial tumors and, despite lowering serum glucose levels, was not associated with improvements in insulin sensitivity or a reduction in intratumoral insulin signaling.
These results are contrary to those of earlier window studies in endometrial cancer, which had almost universally described a significant reduction in cell proliferation with short-term metformin treatment of a similar dose and duration (13–15, 17, 18). These studies had, however, been small, unblinded, nonrandomized and often lacked a control arm for comparison. The major strength of the current trial is its rigorous study design, which included a placebo arm, blinding of participants and the research team, and the use of a standardized, published protocol for determining Ki-67 expression (24). This recommended hotspot scoring as this was the most reliable and reproducible method of quantifying Ki-67 expression and had the closest correlation with traditional pathologic prognostic variables. Earlier endometrial cancer window studies, by contrast, used a whole slide scoring approach; we also undertook whole slide scoring in our study and found it to be highly correlated with hot spot scoring (Pearson correlation coefficient r = 0.91, P < 0.0001), but neither scoring method found an overall effect of metformin on Ki-67 expression.
The absence of an effect of metformin on markers of the PI3K–Akt–mTOR and insulin signaling pathways in this study is in contrast to previously published results and may be explained by the different tumor sampling techniques used to provide posttreatment specimens for analysis. In PREMIUM, an endometrial biopsy was taken immediately prior to the start of hysterectomy and was used for all immunohistochemical secondary outcome measurements rather than the surgical specimen. This removed any effect of surgical clamp-induced tissue hypoxia on marker expression and meant that loss of antigenicity due to delays in fixing large surgical specimens was avoided. We found Ki-67 scores and all secondary tissue endpoints were significantly lower in the resected tumor specimen than in the endometrial sample taken immediately prior to hysterectomy. None, however, showed a significant change with metformin treatment. The reduction in expression of oncogenic signaling proteins previously described in metformin window studies is likely, therefore, to be a sample handling artifact rather than a true effect of the drug, and emphasizes the need for a rigorous control group in such studies (13, 14).
A limitation of the trial was the reliance on surrogate biomarkers of response rather than clinical endpoints, such as reduction in tumor size, grade or cancer-specific survival. Window studies, by their design, utilize the time period between diagnosis and surgical treatment of malignancies to screen treatments for potential therapeutic efficacy more rapidly and at lower cost than traditional adjuvant drug trials. The downside to this is a short and finite treatment period, to avoid compromising patient care, which could mean insufficient time for changes in clinical outcomes. In breast cancer, short-term change in Ki-67 is a validated predictive biomarker of recurrence free survival in women receiving neoadjuvant anastrozole, metformin or both drugs (28). Whilst no studies have directly compared changes in Ki-67 expression with long term clinical outcomes following treatment exposure in endometrial cancer, Ki-67 has been shown to have prognostic value, being associated with cancer specific and recurrence free survival (24). Further longitudinal studies are required to establish its use as a predictive biomarker in endometrial cancer.
The absence of an effect of metformin on endometrial tumor growth described here may be altered with a longer treatment window. The median duration of metformin exposure was similar to that of earlier window studies, but may still be too short. The sample size calculation was based on our earlier pilot study results and assumed an overall reduction in Ki-67 expression with metformin exposure. This meant that it was inadequately powered to look at treatment response in specific subgroups, including stratification according to BMI. Whilst our exploratory subgroup analyses found a possible differential treatment response according to baseline BMI, this association was not substantiated through further interrogation and is likely to be a spurious finding. Our results do not exclude a beneficial effect of metformin in other subgroups of patients with endometrial cancer; future studies should be adequately powered to perform subgroup analyses and undertake more detailed genetic. and molecular characterization of tumors than performed here to aid with this (29)
Although metformin did not show an antiproliferative effect in endometrioid endometrial cancer, it could have clinical utility in other settings. In particular, metformin may have a therapeutic role in non-endometrioid endometrial cancer. Elements of the metabolic syndrome, including obesity and insulin resistance, are associated with increased risk of almost all endometrial cancer subtypes, including high-grade serous and clear cell tumors, and metformin use may confer a survival advantage in diabetic women with nonendometrioid tumors (30, 31). The decision to restrict this study to women with endometrioid endometrial cancer was made because most preclinical and clinical trial data evaluating the role of metformin in endometrial cancer has focused on this cancer subtype. Metformin may also be of benefit in primary disease prevention by improving insulin resistance and reducing the incidence of type 2 diabetes, both major risk factors for endometrial cancer (1). It could be effective in treating atypical endometrial hyperplasia and preventing its recurrence (32), although there is currently insufficient evidence to support its use (33). Preclinical and epidemiologic work have suggested that metformin may act as both a chemo and radiosensitizer (34, 35) and its use alongside radiotherapy and systemic treatments for advanced and recurrent endometrial cancer is currently being tested (36). This latter role for metformin may rely on its specific targeting of cancer stem cells, whose increased migration capacity and resistance to commonly used chemotherapy drugs is thought to be responsible for disease metastasis and relapse (34). Longterm maintenance treatment with metformin to prevent disease recurrence has already been considered in breast cancer and the effect of the drug on 5-year invasive disease-free survival is eagerly awaited (37). Beyond any effect on the course of the disease, metformin may have a role in improving overall survival by treating non-diabetic hyperglycemia and thus reducing the burden of unrecognized risk factors for cardiovascular disease in women newly diagnosed with endometrial cancer (27).
In conclusion, metformin treatment was not associated with anti-proliferative activity, signaling through the PI3K-Akt-mTOR pathways or insulin receptors or inducement of apoptosis when tested in a double-blind, placebo-controlled randomized pre-surgical window trial in endometrioid endometrial cancer. It is, therefore, unlikely to be of value in the primary treatment of the disease.
Supplementary Material
Translational Relevance.
Effective, nonsurgical treatments are required for women who decline or are unfit for hysterectomy for endometrial cancer, and to reduce the risk of relapse. Epidemiologic and preclinical data suggest metformin reduces tumor growth through improvements in insulin sensitivity and the targeting of key carcinogenic pathways in endometrial cancer. Five small, uncontrolled window studies found a reduction in tumor proliferation with short-term metformin but lacked methodological rigor. The PRE-surgical Metformin In Uterine Malignancy (PREMIUM) trial found no effect of standard diabetic doses of metformin on tumor proliferation, activation of the PI3K–AKT–mTOR or insulin signaling pathways in women with endometrial cancer awaiting hysterectomy. These findings highlight the importance of methodologically robust randomized, double-blind, placebo-controlled presurgical window trials that compare matched biopsies before and after intervention and use standardized Ki-67 scoring protocols.
Acknowledgments
We would like to thank all patients who participated in the trial and the clinical staff at recruiting hospitals for their support in conducting this work. We are grateful for the independent members of the Trial Steering Committee, Prof Richard Edmondson, Dr Martin Rutter, Mrs Anne Lowry and Dr Sudha Sundar (Chair of the TSC), for providing trial oversight. E. Crosbie and S. Kitson are funded through a National Institute for Health Research (NIHR) Clinician Scientist Fellowship (NIHR-CS-012-009) and this article presents independent research funded by the NIHR, supported by the NIHR Manchester Biomedical Research Centre and facilitated by the Greater Manchester Local Clinical Research Network. V. Sivalingam is funded through a Wellcome Trust/Wellbeing of Women Research Training Fellowship (ref 098670/Z/12/Z). The views expressed are those of the authors and not necessarily those of the Wellcome Trust, Wellbeing of Women, the NHS, the NIHR or the Department of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
H.C. Kitchener is a consultant/advisory board member for GlaxoSmithKline, related to a HPV therapeutic vaccination unrelated to the topic of this article. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: H.C. Kitchener, E.J. Crosbie
Development of methodology: S.J. Kitson, V.N. Sivalingam, E.J. Crosbie
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S.J. Kitson, Z. Maskell, V.N. Sivalingam, J.L. Allen, S. Ali, S. Burns, K. Gilmour, R. Latheef, R.J. Slade, P.W. Pemberton, J. Shaw, E.J. Crosbie
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S.J. Kitson, W.D. Ryder, H.C. Kitchener, E.J. Crosbie
Writing, review, and/or revision of the manuscript: S.J. Kitson, Z. Maskell, V.N. Sivalingam, J.L. Allen, S. Ali, S. Burns, K. Gilmour, P.W. Pemberton, J. Shaw, W.D. Ryder, H.C. Kitchener, E.J. Crosbie
Study supervision: R.J. Slade, E.J. Crosbie
References
- 1.Kitson SJ, Evans DG, Crosbie EJ. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev Res. 2017;10:1–13. doi: 10.1158/1940-6207.CAPR-16-0224. [DOI] [PubMed] [Google Scholar]
- 2.Evans T, Sany O, Pearmain P, Ganesan R, Blann A, Sundar S. Differential trends in the rising incidence of endometrial cancer by type: data from a UK population-based registry from 1994 to 2006. Br J Cancer. 2011;104:1505–10. doi: 10.1038/bjc.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 4.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–7. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Lopez FR, Pasupuleti V, Gianuzzi X, Palma-Ardiles G, Hernandez-Fernandez W, Hernandez AV. Systematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitus. Maturitas. 2017;101:6–11. doi: 10.1016/j.maturitas.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation–implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, et al. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi: 10.1186/1475-2867-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Barros Machado A, Dos Reis V, Weber S, Jauckus J, Brum IS, von Eye Corleta H, et al. Proliferation and metastatic potential of endometrial cancer cells in response to metformin treatment in a high versus normal glucose environment. Oncol Lett. 2016;12:3626–32. doi: 10.3892/ol.2016.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 10.Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS One. 2013;8:e61537. doi: 10.1371/journal.pone.0061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Li MX, Wang H, Zeng Z, Li XM. Metformin down-regulates endometrial carcinoma cell secretion of IGF-1 and expression of IGF-1R. Asian Pac J Cancer Prev. 2015;16:221–5. doi: 10.7314/apjcp.2015.16.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Xie Y, Wang JL, Ji M, Yuan ZF, Peng Z, Zhang Y, et al. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncology letters. 2014;8:1993–9. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer. 2014;120:2986–95. doi: 10.1002/cncr.28853. [DOI] [PubMed] [Google Scholar]
- 14.Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol Oncol. 2014;134:607–14. doi: 10.1016/j.ygyno.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Soliman PT, B R, Westin SN, Iglesias DA, Burzawa JK, Zhang Q, Munsell, et al. Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer: A window of opportunity study. J Clin Oncol. 2014;32:5510. doi: 10.1016/j.ygyno.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Med. 2015;4:161–73. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer. 2016 doi: 10.1038/bjc.2015.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Sun H, Feng M, Zhao J, Zhao X, Wan Q, et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol Endocrinol. 2017:1–5. doi: 10.1080/09513590.2017.1409714. [DOI] [PubMed] [Google Scholar]
- 19.Pinhel IF, Macneill FA, Hills MJ, Salter J, Detre S, A' Hern R, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12:R76. doi: 10.1186/bcr2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emons G, Beckmann MW, Schmidt D, Mallmann P, Uterus commission of the Gynecological Oncology Working G New WHO Classification of Endometrial Hyperplasias. Geburtshilfe und Frauenheilkunde. 2015;75:135–6. doi: 10.1055/s-0034-1396256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23:232–54. doi: 10.1093/humupd/dmw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program and Common Terminology Criteria for Adverse Events. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) 2009 Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 23.Nielsen F, Christensen MM, Brosen K. Quantitation of metformin in human plasma and urine by hydrophilic interaction liquid chromatography and application to a pharmacokinetic study. Ther Drug Monit. 2014;36:211–7. doi: 10.1097/FTD.0b013e3182a4598a. [DOI] [PubMed] [Google Scholar]
- 24.Kitson S, Sivalingam VN, Bolton J, McVey R, Nickkho-Amiry M, Powell ME, et al. Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol. 2017;30:459–68. doi: 10.1038/modpathol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat. 2014;148:81–90. doi: 10.1007/s10549-014-3141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. Waist circumference and waist-hip ratio. Report of a WHO expert consultation. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 27.Kitson SJ, Lindsay J, Sivalingam VN, Lunt M, Ryan NAJ, Edmondson RJ, et al. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: A prospective case control study. Gynecol Oncol. 2018;148:154–60. doi: 10.1016/j.ygyno.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–8s. [PubMed] [Google Scholar]
- 29.Lord SR, Cheng WC, Liu D, Gaude E, Haider S, Metcalf T, et al. Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metab. 2018;28:679–88. doi: 10.1016/j.cmet.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabert B, Wentzensen N, Felix AS, Yang HP, Sherman ME, Brinton LA. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev. 2015;24:261–7. doi: 10.1158/1055-9965.EPI-14-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132:236–40. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancerdagger. Ann Oncol. 2016;27:262–6. doi: 10.1093/annonc/mdv539. [DOI] [PubMed] [Google Scholar]
- 33.Clement NS, Oliver TR, Shiwani H, Sanner JR, Mulvaney CA, Atiomo W. Metformin for endometrial hyperplasia. Cochrane Database Syst Rev. 2017;10:CD012214. doi: 10.1002/14651858.CD012214.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezewuiro O, Grushko TA, Kocherginsky M, Habis M, Hurteau JA, Mills KA, et al. Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy. PLoS One. 2016;11:e0147145. doi: 10.1371/journal.pone.0147145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials gov. Paclitaxel and carboplatin with or without metformin hydrochloride in treating patients with stage III, IV, or recurrent endometrial cancer. 2016 Available from: https://clinicaltrials.gov/ct2/show/NCT02065687?term=metformin+AND+endometrial+cancer&rank=4.
- 37.Goodwin PJ, Parulekar WR, Gelmon KA, Shepherd LE, Ligibel JA, Hershman DL, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.