Abstract
Background and Aim
Systemic sclerosis (SSc) is known to involve the gastrointestinal (GI) tract, resulting in dysmotility, gastroesophageal reflux disease, and mucosal changes causing significant morbidity. The study aimed to assess esophageal motility and duodenal mucosal changes in SSc.
Methods
This is a prospective, cross‐sectional, single‐center study of 23 patients with SSc diagnosed on the basis of standard criteria. Clinical details including the GI symptoms were recorded. All of them underwent esophagogastroduodenoscopy with duodenal biopsy, and 21 underwent esophageal manometry.
Results
Regurgitation, heartburn, and dysphagia were seen in 19 (82%), 16 (69%), and 10 (43%) patients, respectively. On endoscopy, 19 patients (83%) showed changes of reflux esophagitis (4 had grade C esophagitis), and 3 had esophageal candidiasis. Of the 21 patients who underwent esophageal manometry, 13 (62%) had absent peristalsis, 6 (28%) had ineffective peristalsis, and 10 (48%) had hypotensive lower esophageal sphincter (LES). Duodenal biopsy showed partial villous atrophy in 9 (39%) patients, increased intraepithelial lymphocytes in 18 (78%), and excess of mononuclear inflammatory cells in lamina propria in 21 (91%). Partial villous atrophy was seen more commonly in those with abnormal esophageal peristalsis and a hypotensive LES.
Conclusion
Most of the patients with SSc had esophageal dysmotility in the form of absent peristalsis, ineffective esophageal peristalsis, and hypotensive LES. Histology of descending duodenum demonstrated partial villous atrophy and chronic inflammatory infiltrates in most of the patients with SSc.
Keywords: celiac disease, gastrointestinal, manometry, scleroderma, systemic sclerosis, villous atrophy
Introduction
Systemic sclerosis (SSc) is an autoimmune disease that is characterized by abnormalities of microvasculature with increased collagen and other connective tissue component deposition in skin and internal organs.1 Gastrointestinal (GI) involvement is the most frequent visceral complication, with motility disorders and reflux esophagitis adding to the morbidity. GI mucosal changes have been reported only rarely in literature. The aim of this study was to prospectively evaluate esophageal and gastroduodenal involvement in a cohort of ambulatory SSc patients. Special emphasis was given to duodenal mucosal histopathological changes and their correlation with esophageal and duodenal endoscopic and manometric changes as data are limited in our population.
Methods
This is a prospective, observational, single‐center study that included patients with SSc who fulfilled the American College of Rheumatology (ACR) criteria of 1983 and who attended the rheumatology clinic of a tertiary care center in North India. Patients with overlap syndrome, diabetes mellitus, and who underwent abdominal surgeries were excluded from the study. Baseline demographic data and clinical features with emphasis on the GI system were collected. Patients were classified into limited cutaneous and diffuse cutaneous based on LeRoy classification.2
All recruited patients underwent esophagogastroduodenoscopy with concurrent duodenal biopsy after 8 h of overnight fasting. Esophagogastroduodenoscopy was performed by an expert gastroenterologist using video endoscopes (Olympus GIF 150/160/180). Detailed examinations of the esophagus, stomach, duodenal bulb, and descending duodenum were carried out, and multiple random duodenal biopsies were taken. The samples were preserved in neutral formalin for histological examination and were examined by an expert GI histopathologist who was unaware of the clinical details. Duodenal biopsy was classified according to the modified Marsh‐Oberhuber classification.3
Twenty‐one patients underwent esophageal manometry. A water‐perfused system (from Medical Measurement System, Sweden) using a six‐ or eight‐channel catheter was used for esophageal manometry. Peak amplitudes of waves were measured 3 and 8 cm above the lower esophageal sphincter (LES), with nature of wave form, type of contraction waves (peristaltic, simultaneous, retrograde, or nontransmitted), wave velocity, resting LES pressure, percentage relaxation of LES in response to wet swallows, and residual LES pressure being assessed. Measurements were taken for at least 10 wet swallows. Manometry was considered abnormal if the peristaltic waves were absent or ineffective (ineffective if the peak amplitude reached is less than 30 mmHg in more than 20% of swallows) or LES pressure was below 10 mmHg. Patients underwent the above examinations independent of the presence of GI complaints.
Written informed consent was obtained from the patients, and the study was approved by the institute's ethics committee. Statistical analysis was carried out using SPSS version 20, IBM, USA. Normality was checked using measures of Kolmogorov–Smirnov tests of normality. For normally distributed data, means were compared using the t‐test and the Mann–Whitney test for skewed data. Proportions were analyzed using χ 2 or Fisher's exact test. All statistical tests were two sided, and a P value ≤0.05 was considered significant in all statistical evaluations.
Results
Twenty‐three patients with a mean age of 35.7 ± 12.7 years were included in the study. Twenty patients were female, and three were male; 16 patients had limited cutaneous disease. The mean skin score was 20.5 ± 7.7, and mean duration of GI symptoms was 2.7 years. Baseline characteristics are given in Table 1. All patients had skin manifestations in the form of sclerodactyly and skin pigmentation. Four patients had CREST syndrome (calcinosis, Raynaud's phenomenon, esophageal involvement, sclerodactyly, and telangiectasia). On esophagogastroduodenoscopy, esophagitis was observed in 19 patients (83%). Of them, four had grade C esophagitis, and three had esophageal candidiasis; antral hyperemia was seen in three and antral erosions and antral polyp in 1. The duodenum appeared macroscopically normal in all patients.
Table 1.
Baseline characteristics
| Group/variables | SSc (n = 23) | lcSSc (n = 16) | dcSSc (n = 7) |
|---|---|---|---|
| Mean age (years) | 35.7 ± 12.7 | 36.5 ± 13.6 | 33.7 ± 11.1 |
| Female: male | 20:3 | 14:2 | 6:1 |
| Mean mRSS | 20.5 ± 7.7 | 17.5 ± 3.8 | 27.5 ± 10.0 |
| Mean BMI | 21.3 ± 3.0 | 21.4 ± 3.0 | 21.3 ± 3.2 |
| Duration of GI symptoms in years (median, IQR) | 2 (0.5, 4.0) | 2.5 (0.5, 4.75) | 1.0 (1.0, 4.0) |
| Duration of skin tightening in years (median, IQR) | 3.0 (1.5, 6.0) | 4.0 (2.0, 8.25) | 2.0 (1.0, 5.0) |
| Raynaud's phenomenon | 23 | 16 | 7 |
| Sclerodactyly | 23 | 16 | 7 |
| Digital pitting scars | 21 | 15 | 6 |
| Skin pigmentation | 23 | 16 | 7 |
| Telengiectasia | 11 | 9 | 2 |
| Digital infarct | 6 | 3 | 3 |
| Calcinosis | 5 | 5 | 0 |
| Regurgitation | 19 | 12 | 7 |
| Heartburn | 16 | 10 | 6 |
| dysphagia | 10 | 6 | 4 |
| Mean hemoglobin (mg/dL) | 11.2 ± 1.8 | 10.9 ± 1.9 | 11.9 ± 1.2 |
| Mean albumin (g/dL) | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.3 ± 0.4 |
BMI, body mass index; dcSSc, diffuse cutaneous; GI, gastrointestinal; IQR, interquartile range; lcSSc, limited cutaneous; mRSS, modified Rodnan skin score; SSc, systemic sclerosis.
Of the 21 patients for whom esophageal manometry was performed, hypotensive LES was seen in 10 (48%), an absent peristalsis in 13 (62%), and ineffective peristalsis in 6 (28%). The average LES pressure was 15 ± 9.2 mmHg.
Duodenal biopsy findings are summarized in Table 2. The most common finding was an excess of mononuclear inflammatory cells in lamina propria, which was seen in 21 patients (91%), while 9 (39%) had partial villous atrophy (Fig. 1a). Giardiasis and dilated lymphatics were found in one patient. Normal histology was seen in only one patient (Fig. 1b). All patients with villous atrophy had crypt hyperplasia, increased intra epithelial lymphocytes, and excess of mononuclear inflammatory cells in lamina propria. Partial villous atrophy was higher in those with a hypotensive LES (P = 0.03), but it had no statistically significant correlation with symptoms like abdominal distension (P = 0.21), regurgitation (P = 0.10), abdominal pain (P = 0.9), or diarrhea (P = 0.5) or with the presence of esophagitis (P = 0.9), digital infarct (P = 0.3), or telangiectasia (P = 0.2). The mean hemoglobin was 11.2 ± 1.8 gm/dL, with 10 patients having hemoglobin less than 11 gm/dL. Mean body mass index (BMI) was 21.3 ± 3.0. No patient had hypoalbuminemia or hypocalcemia.
Table 2.
Duodenal biopsy findings in the study group
| SSc (n = 23) | lcSSc (n = 16) | dcSSc (n = 7) | |
|---|---|---|---|
| Histological changes | |||
| No villous architecture alteration | 14 | 10 | 4 |
| Villous architecture alterations | 9 | 6 | 3 |
| Increased intra epithelial lymphocytes | 18 | 12 | 6 |
| Lamina propia infiltrate in excess | 21 | 14 | 7 |
| Crypt hyperplasia | 18 | 13 | 5 |
| Histological diagnosis | |||
| Normal morphology | 1 | 1 | 0 |
| Intraepithelial lymphocytosis without crypt hyperplasia | 3 | 2 | 1 |
| Partial villous atrophy | 9 | 6 | 3 |
| Nonspecific changes | 8 | 6 | 2 |
| Acute duodenitis | 2 | 1 | 1 |
dcSSc, diffuse cutaneous; lcSSc, limited cutaneous; SSc, systemic sclerosis.
Figure 1.
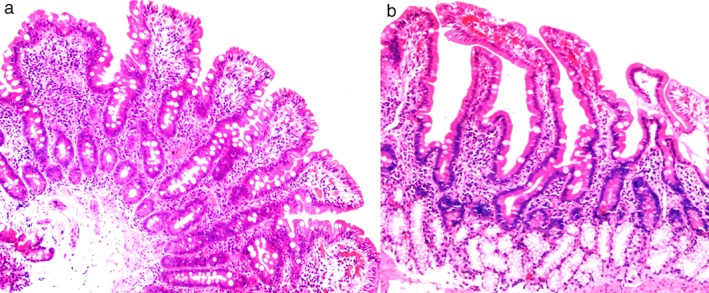
(a) Duodenal mucosa showing partial villous atrophy in systemic sclerosis (HE ×100). (b) Normal duodenal mucosa in systemic sclerosis (HE ×100).
Discussion
GI involvement was seen in all patients in the study, either clinically or in the investigations. An increased incidence of duodenal villous atrophy was seen in the study population in addition to esophageal dysmotility and esophagitis. In fibrotic stages of SSc, degeneration of epithelial cells and flattening of epithelium, along with subepithelial fibrosis and atrophy of muscularis mucosa, are observed.4 Significant thickening of the walls of the upper GI tract with predominant involvement of the submucosa and muscularis has also been demonstrated on endoscopic ultrasound.5 These results support the hypothesis that increased matrix deposition is important in the pathogenesis of GI involvement. In the present study, partial villous atrophy and increased epithelial lymphocytes were seen more commonly in those with abnormal esophageal peristalsis and a hypotensive LES. This may suggest that these histological changes occur as a part of the disease rather than a separate entity. However, the presence of villous atrophy had no correlation with esophagitis or any GI symptoms. In the study by Cobden et al., where they assessed small intestinal structure and passive permeability in 17 patients with proven SSc by peroral jejunal biopsy, abnormalities were seen only in 4 patients and were mostly confined to the deeper structures.6 Minimal degree of villous atrophy without epithelial cell changes were seen in two. Compared to them, in our study, villous atrophy was seen in nearly one third of the patients.
Villous atrophy in SSc has been linked to celiac disease by some authors. There are case reports of SSc with celiac disease.7 Although Rosato et al. proposed an association between celiac and SSc, with a prevalence of 8%, this was later refuted by Forbess et al. 8, 9 The baseline prevalence of celiac disease in our population ranges from 0.33 to 1.04.10, 11 Hence, it is reasonable to think that there is a significantly high incidence of villous atrophy in SSc patients. Other causes of villous atrophy include common variable immunodeficiency (CVID), autoimmune enteropathy, small intestinal bacterial overgrowth (SIBO), infection, intestinal lymphoma, collagenous sprue, Crohn's disease, and tropical sprue.12 In our study, except for one patient with giardiasis, no such causes were found. As we have not looked for serological evidence for celiac and autoimmune enteropathy, they cannot be excluded as a cause.
It was seen in the study that the presence of partial villous atrophy had no statistically significant correlation with anemia, hypocalcemia, hypoproteinemia, or low creatinine levels to suggest malnutrition among this subset of patients. It has been previously shown that SIBO can lead to malabsorption and malnutrition in SSc. Loss of villi and microvilli in the small bowel has been cited as one reason for the malabsorption syndrome in SSc patients.13 In our study, the presence of these histological changes did not have any correlation with any GI symptom, similar to what was seen with study cohort of Lindsy et al. 9 Neither the type of SSc nor the Rodnan score had any association with the occurrence of villous atrophy.
There are few limitations to the study. The number of subjects was lower to draw a more meaningful conclusion. Other causes of villous atrophy have not been looked for in the study to clearly state that the changes are because of SSc alone.
In conclusion, most of our patients with SSc had esophageal dysmotility in the form of absent peristalsis, ineffective esophageal peristalsis, and hypotensive LES. Compared to Western population, histology of the descending duodenum demonstrated partial villous atrophy and/or chronic inflammatory changes in most of the patients with SSc.
Acknowledgment
Indian Rheumatology Association—Scholar's retreat 2.0.
Declaration of conflict of interest: All authors have none to declare.
Financial support: The study was performed in a government hospital using existing infrastructure. No outside funding was taken.
References
- 1. Prescott RJ, Freemont AJ, Jones CJP, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992; 166: 255–63. [DOI] [PubMed] [Google Scholar]
- 2. LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001; 28: 1573–6. [PubMed] [Google Scholar]
- 3. Prasad KK, Thapa BR, Nain CK, Sharma AK, Singh K. Brush border enzyme activities in relation to histological lesion in pediatric celiac disease. J. Gastroenterol. Hepatol. 2008; 23: e348–52. [DOI] [PubMed] [Google Scholar]
- 4. Sallam H, Mcnearney TA, Chen JDZ. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma). Aliment. Pharmacol. Ther. 2006; 23: 691–712. [DOI] [PubMed] [Google Scholar]
- 5. Zuber‐Jerger I, Müller A, Kullmann F et al. Gastrointestinal manifestation of systemic sclerosis—thickening of the upper gastrointestinal wall detected by endoscopic ultrasound is a valid sign. Rheumatology. 2010; 49: 368–72. [DOI] [PubMed] [Google Scholar]
- 6. Cobden I, Rothwell J, Axon AT, Dixon MF, Lintott DJ, Rowell NR. Small intestinal structure and passive permeability in systemic sclerosis. Gut. 1980; 21: 293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez‐Puerta JA. Coeliac disease associated with systemic sclerosis. Ann. Rheum. Dis. 2004; 63: 104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosato E, De Nitto D, Rossi C et al. High incidence of celiac disease in patients with systemic sclerosis. J. Rheumatol. 2009; 36: 965–9. [DOI] [PubMed] [Google Scholar]
- 9. Forbess LJ, Gordon JK, Doobay K et al. Low prevalence of coeliac disease in patients with systemic sclerosis: a cross‐sectional study of a registry cohort. Rheumatology (Oxford). 2013; 52: 939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sood A, Midha V, Sood N, Avasthi G, Sehgal A. Prevalence of celiac disease among school children in Punjab, North India. J. Gastroenterol. Hepatol. 2006; 21: 1622–5. [DOI] [PubMed] [Google Scholar]
- 11. Makharia GK, Verma AK, Amarchand R et al. Prevalence of celiac disease in the northern part of India: a community based study. J. Gastroenterol. Hepatol. 2011; 26: 894–900. [DOI] [PubMed] [Google Scholar]
- 12. Pallav K, Leffler DA, Tariq S et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment. Pharmacol. Ther. 2012; 35: 380–90. [DOI] [PubMed] [Google Scholar]
- 13. Kumar V, Robbins SL. Robbins Basic Pathology, 8th edn. Philadelphia, PA: Saunders/Elsevier, 2008. [Google Scholar]


