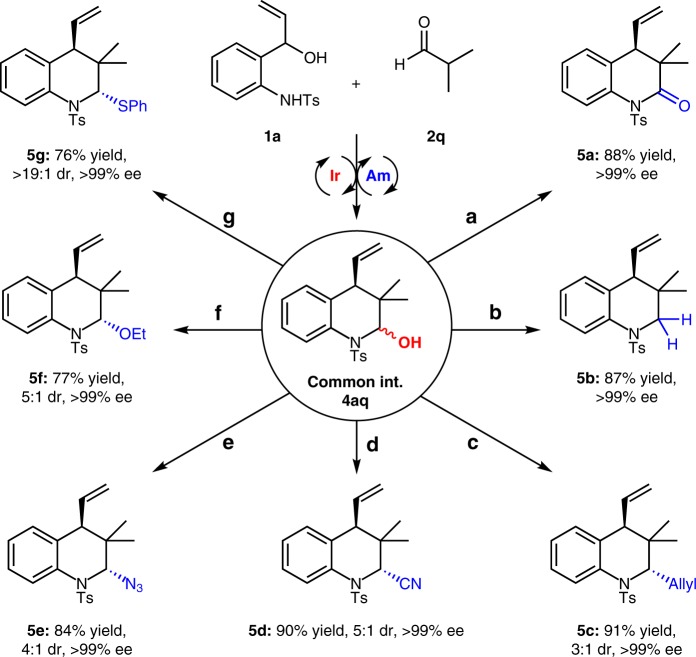
Fig. 4.
Divergent synthetic transformations of chiral hemiaminal 4aq. Reaction conditions: a) PCC (5.0 eq.), silica (100 mg), CH2Cl2 (5 mL), 40 °C, 10 h; b) Et3SiH (3.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; c) allyltrimethylsilane (3.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; d) TMSCN (2.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; e) TMSN3 (2.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; f) p-TSA (20 mol%), EtOH (2.5 mL), 30 °C, 10 h. g) PhSH (1.5 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; isolated yield; the ee and dr values were determined by chiral HPLC analysis of purified products and 1H NMR analysis of reaction mixtures, respectively. p-TSA: p-toluenesulfonic acid