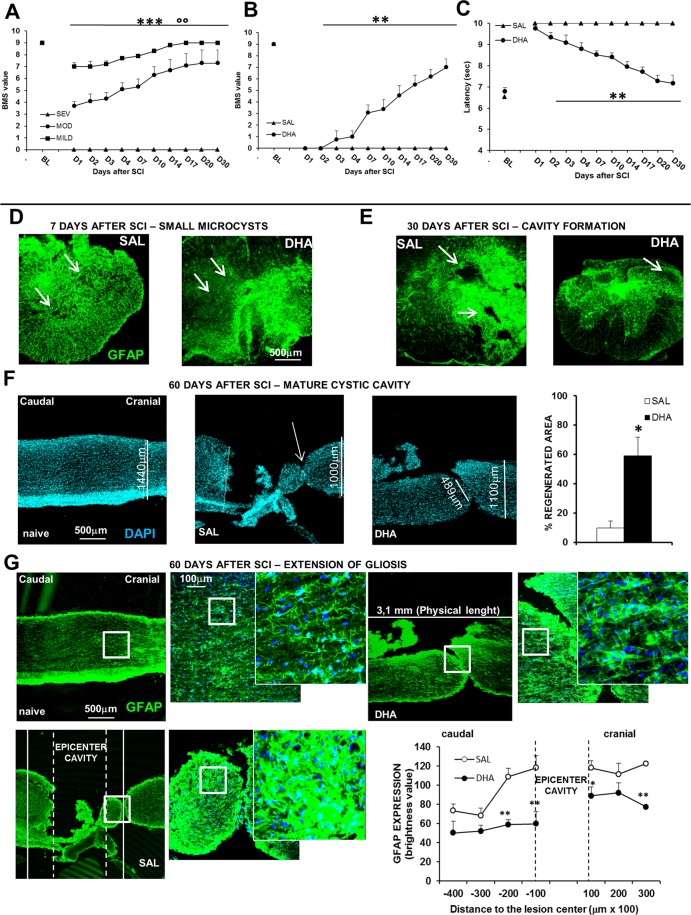
Figure 2.
Behavioural responses, microcystic degeneration and cystic cavitation in the new mouse model of SCI. (A) BMS scores in female mice subjected to different degrees of impact. BMS defines the locomotion in recovery after SCI. The BMS score ranges from 0 to 9, where 0 indicates complete paralysis and 9 normal movement of the hindlimbs. Motor function recovery is significantly different among the three degree of impact (°°p < 0.01 mild vs moderate; ***p < 0.001 mild and moderate vs severe SCI, Tukey/Kramer; n = 5 mice/group). (B) BMS scores and (C) thermal threshold in daily ip saline- (SAL) or DHA-injected (from D1 to D5 after SCI) females. DHA-treated animals (n = 8) show a significant improvement in motor function, as revealed by higher BMS values in comparison with SAL-treated animals (n = 11) (***p < 0.001), and a significant recovery of tail-flick reflex (***p < 0.001) (Tukey/Kramer). (D) Representative images (5X) of small microcysts, as revealed by GFAP (green) immunostaining, 7 days after SCI in SAL- and DHA-treated mice. (E) Representative image (5X) of cavity formation 30 days after SCI in SAL- and DHA-treated mice. (F) Nuclei stained with DAPI in longitudinal representative images (5X) of spinal cord in naïve, SAL- and DHA-treated mice, 60 days after SCI; DHA induced significant (**p < 0.01) beneficial effects, as shown by the percentage increase of regenerated area in the graph (n = 3 mice/group). (G) GFAP (green) staining in longitudinal representative images (5X) of spinal cord in naïve, SAL- and DHA-treated mice 60 days after SCI. The squares represented high magnification images (20x and zoom 2 respectively). The areas 200 μm caudal and cranial in respect to the epicenter cavity are quantified in the graph. Note, in the graph, that DHA significantly reduced GFAP expression at caudal and cranial level (n = 4 sample/group) (*p < 0.05, **p < 0.01 vs SAL Student’s t test).