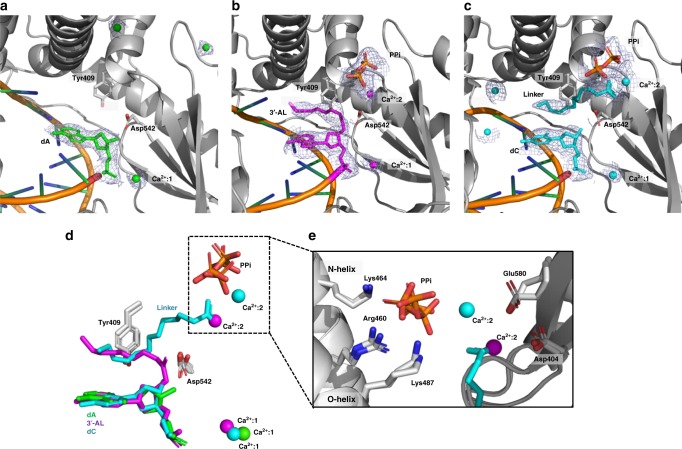
Fig. 2.
Structural evidence for the 3′-esterase activity of 9°N-I DNA polymerase. a The crystal structure of 9°N-I in complex with primer/template (P/T) duplex DNA with dATP incorporation. Simulated annealing 2Fo-Fc omit maps (light gray) centered on the translocated dAMP (green) and Ca2+ (green), and contoured at 1.0 σ are shown. The active-site residues Tyr409 and Asp542 are also indicated. b Same with a, except with 3′-AL incorporation, and centered on the incorporated monophosphate 3′-AL (magenta), PPi (orange) and Ca2+ (magenta), contoured at 0.8 σ. c Same with a, except with 3′-CL incorporation, centered on the translocated dCMP (cyan), cleaved 3′-linker moiety (cyan), PPi (orange) and Ca2+ (cyan), contoured at 1.0 σ. d Superimposed stick model of the incorporated nucleotides, PPi, residues Tyr409 and Asp542, and Ca2+ in the active site of 9°N-I from a to c. e Close-up view of the PPi interaction network from d