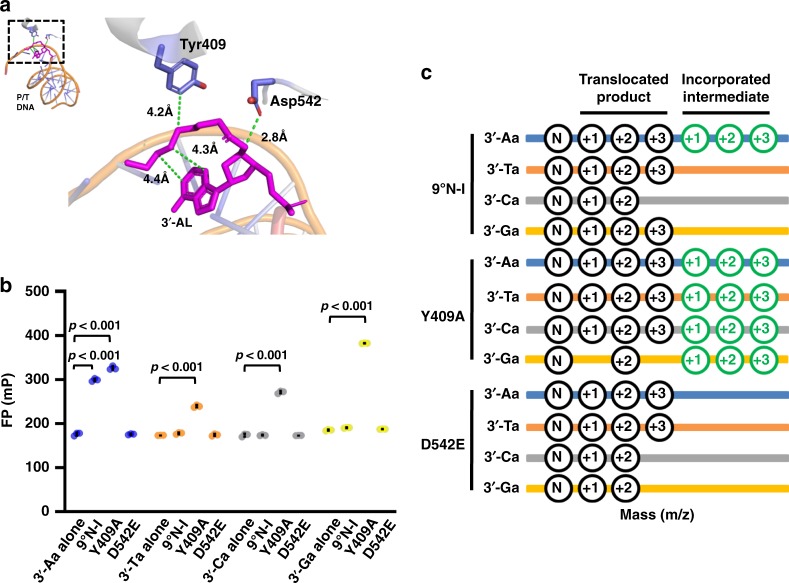
Fig. 4.
The esterase activity is modulated by active site residues. a Involvement of Tyr409 and Asp542 at the active site of 9°N-I in the cleavage of 3′-esterified nucleotide. The distance between 3′-esterified moiety of 3′-AL (colored in magenta) and Tyr409 or Asp542 (colored in blue) are shown. b FP activity of 3′-Na (including 3′-Aa, 3′-Ta, 3′-Ca, and 3′-Ga) after incorporation into the primer by 9°N-I and its mutants Y409A and D542E (n = 3). The primer extension was performed with dN3-based templates. c MALDI-TOF/TOF MS profiles of the incorporated intermediates of 3′-Na catalyzed by both Y409A and D542E. After primer extension, the detected ester intermediates and translocated products (+1, +2, and +3) were colored in green and black, respectively. Details are described in Supplementary Figs. 8–10