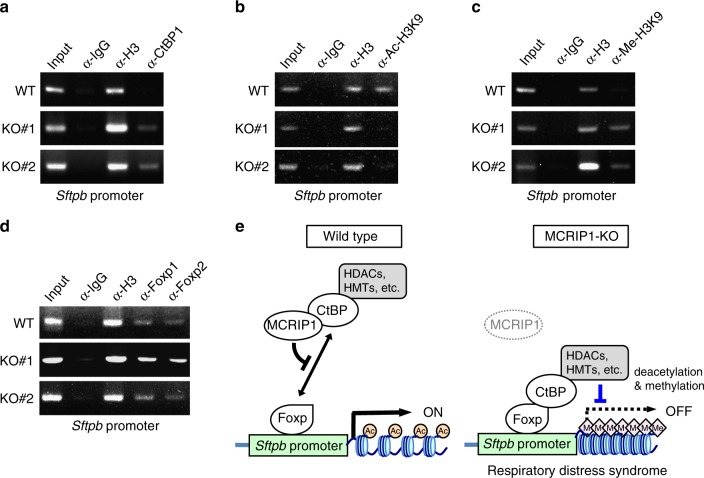
Fig. 5.
MCRIP1 depletion allows the recruitment of a CtBP co-repressor complex to the SP-B promoter and modulates histone modifications. a–d Chromatin immunoprecipitation (ChIP) assays were performed using parental (WT) and Mcrip1-KO (KO#1 and KO#2) MLE12 cells. Cross-linked chromatin was immunoprecipitated with antibodies specific for CtBP1 a, histone H3 acetylated at Lys9 (Ac-H3K9) b, histone H3 methylated at Lys9 (Me-H3K9) c, or Foxp1, or Foxp2 d. Immunoprecipitated DNAs were amplified with PCR primers specific for the SP-B promoter. Rabbit IgG and an anti-Histone H3 antibody were used as negative and positive controls, respectively. The input lanes represent 2% of the total chromatin used for each immunoprecipitation. e A schematic model of MCRIP1-dependent epigenetic regulation of SP-B and SP-C expression. In the lung epithelial cells of Mcrip1+/+ (wild-type) mice, MCRIP1 binds to CtBP and thereby disrupts the interaction of CtBP with DNA-binding transcriptional repressors (e.g., Foxp1/2). As a result, CtBP cannot be recruited to the promoters of target genes (e.g., SP-B and SP-C). In contrast, in Mcrip1−/− (MCRIP1-KO) lungs, the absence of MCRIP1 allows CtBP to interact with Foxp1/2 on the promoter regions of SP-B and SP-C. When bound to the promoters, the CtBP co-repressor complex, which includes histone-remodeling enzymes (e.g., HDACs and HMTs), modulates chromatin conformation via deacetylation and methylation of histones, resulting in transcriptional silencing of the surfactant proteins and the induction of respiratory failure that resembles human infant RDS