Figure 5.
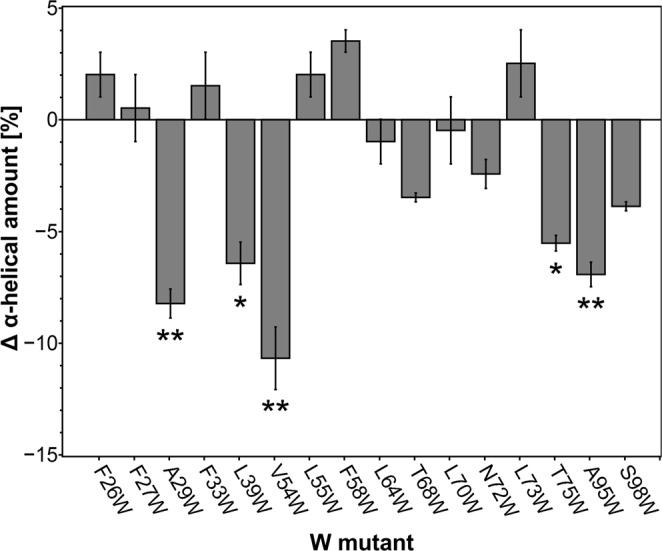
Changes in overall α-helical content in ETR1 by tryptophan substitution. Secondary structure content of purified full-length ETR1 tryptophan mutants was determined by CD spectroscopy. Changes in α-helical content related to individual tryptophan mutants are shown as deviation from the tryptophan-free background mutant ETR1W7X. All measurements were taken in triplicates. Mean and standard deviation are shown (*P ≤ 0.05; **P ≤ 0.02).
