Figure 5.
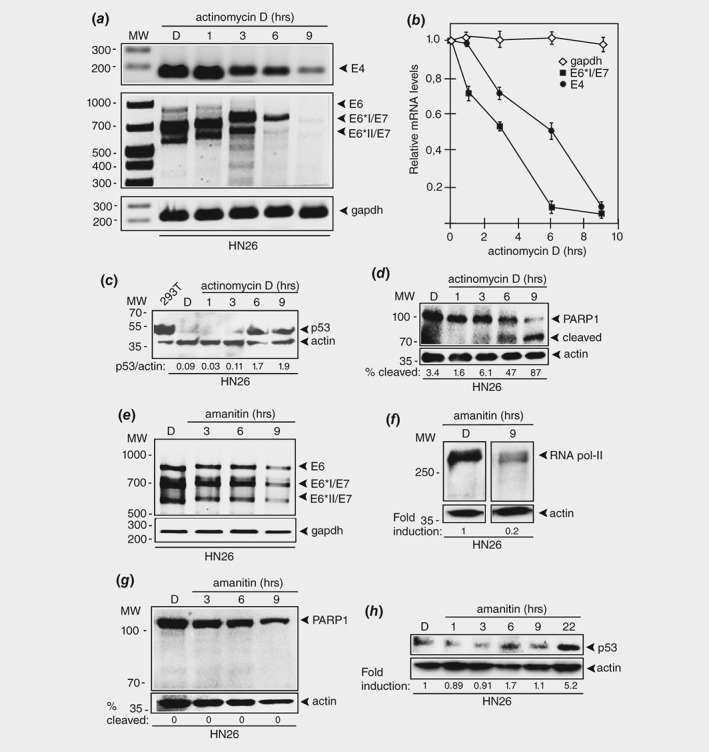
Actinomycin D causes rapid degradation of HPV16 E6 and E7 oncogene mRNAs in HPV16 positive tonsillar cancer cells. (a) RT‐PCR on total RNA extracted from HN26 cells treated with DMSO alone or 1.5 μM of actinomycin D for the indicated time periods. HPV16 E4, E6, E6*I/E7 and E6*II/E7 mRNAs were monitored as well as spliced cellular gapdh mRNA. The location of the RT‐PCR primers in the HPV16 genome is shown in Supporting Information Figure 5B. (b) The RT‐PCR bands representing HPV16 mRNAs were quantified and plotted against hours of 1.5 μM actinomycin D treatment of the HN26 cells. (c) Western blot with monospecific antibody to p53 on extracts from 293T cells or HN26 cells treated with DMSO (D) or actinomycin D for the indicated time points. (d) Western blot with monospecific antibody to PARP1 on extracts from HN26 cells treated with DMSO (D) or 1.5 μM actinomycin D for the indicated time points. (e) RT‐PCR on total RNA extracted from HN26 cells treated with DMSO alone or 3 μg/mL of alpha‐amanitin for the indicated time periods. HPV16 E6, E6*I/E7 and E6*II/E7 mRNAs were monitored as well as spliced cellular gapdh mRNA. (f) Western blot on extracts from HN26 cells incubated with DMSO (D) or 3 μg/mL of alpha‐amanitin for 9 h with monospecific antibody to RNA polymerase II and actin. (g) Western blot with monospecific antibody to PARP1 on extracts from HN26 cells treated with DMSO (D) or 3 μg/mL alpha‐amanitin for the indicated time points. (H) Western blot with monospecific antibody to p53 on extracts from 293T cells or HN26 cells treated with DMSO (D) or alpha‐amanitin (3 μg/mL) for the indicated time points.
