Abstract
Background:
The diabetic kidney disease (DKD) has become a seriously kidney disease that commonly caused by diabetes mellitus (DM). Oxidative stress response plays an essential role in the genesis and worsening of DKD and Coenzyme Q10 (CoQ10) has been reported the promising clinical effectiveness on DKD treatment. However, there is lack of relative evidence-based medical evidence currently.
Objective:
The systematic review and meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, which conducted to evaluate the effectiveness of CoQ10 in combination with other western medicine for DKD therapy through the randomized controlled trials (RCTs) and experimental studies.
Methods:
RCTs and experimental studies were searched based on standardized searching rules in 12 medical databases from the inception up to June 2018 and a total of 8 articles (4 RCTs and 4 experimental studies) were enrolled in the meta-analysis.
Results:
The results revealed that CoQ10 combined with other western medicine show statistical differences in the laboratory parameters of fasting plasma glucose (FPG), Hemoglobin A1c (HbA1c), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), triglyceride (TG), and malondialdehyde (MDA) amelioration after DKD therapy compared with control group. However, LDL-C and Urea level for RCTs and Urine output and Glucose for experimental studies on DKD was not superior to control group.
Conclusion:
We need to make conclusion cautiously for the effectiveness of CoQ10 application on DKD therapy. More standard, multicenter, double-blind RCTs, and formal experimental studies of CoQ10 treatment for DKD were urgent to be conducted for more clinical evidence providing in the future. The underlying pharmacological mechanism of CoQ10 needs to be researched and revealed for its future application on DKD therapy.
Keywords: diabetes mellitus, diabetic kidney disease, nephrology, therapy
1. Introduction
As one of essential complications of diabetes mellitus (DM), diabetic kidney disease (DKD) has become a heavy burden on patients, leading the chronic loss of kidney function and increasing the risk of chronic kidney disease (CKD) and end-stage renal disease (ESRD) dramatically.[1–3] About 30% to 50% of diabetic patients in the United States would progress into ESRD in the end and about 30% to 40% diabetic patients would develop into DKD based on the latest guidelines.[4] In China about 114 million adult patients with DM that account for nearly 11.7% of total populations, making the country become the fastest growing one in the world for DM.[5] The Chinses ministry of health particular highlights that the DKD has become the leading cause of ESRD in China.[6]
The kidney damage of DKD consists of glomeruli, renal tubules, renal interstitium, and renal blood tube, emphasizing the overall damage of DM in the kidney.[7] As for the disease natural development, it should be noticed that the greatest predictor of renal function deterioration and DKD progression is the proteinuria.[4] The diagnosis of DKD can be divided as 2 parts, clinic and pathology. Although the clinical diagnosis mainly based on the urinary albumin excretion (UAE) and diabetic retinopathy (DR),[8] nowadays it is essential to emphasize early screening the high risk population of DKD.[9] The pathological diagnosis, which considered as the golden criterion of DKD, is the glomerular abnormalities caused by DM manifested as the glomerular basement membrane (GBM) thickening, the mesangial expansion and the glomerulosclerosis with Kimmelstein-Wilson nodules.[10,11] However, there is still a lack of clinical evidence to clearly make sure the specific stage of renal biopsy for patients with DM.[11] The treatment of patients with DKD can be divided into 4 parts: the control and management of blood glucose, cardiovascular disease risk, blood pressure, and renin-angiotensin system (RAS).[4] The goal of glycemic control for T1DM and T2DM was to reduce the HbA1c level to 7.0%, reducing the microvascular risks and DKD progression.[12,13] Any further reduction of blood glucose may put the patient at risk of hypoglycemic events.[12] Treatments that reduce the risk of cardiovascular, including smoking cessation and lipid-lowering, lack clinical evidence to prove the clinical efficacy for DKD.[14,15] According to the latest guideline, the blood pressure of DKD should be <140/90 mmHg.[4] The inhibition of RAS therapy has proven to be the most effective monotherapy to slow down the DKD progression, even though it has clinical adverse events (such as hyperkalemia, acute kidney injury, and increased cardiovascular events) and cannot prevent the ESRD emerges.[16,17] There were some emerging therapies applied in clinical practices such as the finerenone (third-generation mineralocorticoid receptor antagonist) and sodium glucose cotransporter 2 (SGLT2) inhibitors, focusing on the molecular mechanism of inflammation, fibrosis, and extracellular matrix deposition.[18,19]
Coenzyme Q10 (CoQ10) has been proved its essential role in generating adenosine triphosphate (ATP) in the chain of mitochondrial respiratory, reducing the reactive oxygen species (ROS) and activating the mitochondrial dehydrogenases and enzymes.[20] Numerous reviews and trials have recognized the clinical potential of CoQ10 to manage and control the cardiovascular diseases (CVD),[21,22] DM,[23] and hypercholesterolemia.[24] Besides that, CoQ10 has the promising clinical effectiveness because the oxidative stress response plays the essential role in DKD even though it needs further clinical assessment.[25] However, there is still a lack of evidence-based medical evidence for the clinical treatment of DKD with CoQ10 and a meta-analysis review questioning the clinical efficiency of antioxidants for DKD.[26] We therefore conducted a systematic review and meta-analysis of clinical trials and experimental studies to investigate whether CoQ10 may represent a potential therapy for DKD.
2. Methods
Sine this study is a meta-analysis of previously published studies, the ethical approval and patient consent are not required.
This systematic review and meta-analysis was based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[27]
2.1. Data sources and support
The international mainstreamed medical electronic databases including PubMed, Web of Science, Ovid-Medline, ProQuest, Science Direct, Springer link, Wiley Library Online, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chinese medical Citation Index (CMCI), Chinese Scientific Journal Database (VIP), and Wanfang database were selected for articles searching from their inception up to June 2018 by 3 reviewers’ (ZS, QL, and XZ) mutual cooperation. We did not make any language or time restrictions on literature searching in the selected database.
2.2. Search protocol
The searching protocol was conducted as followed: “coenzyme Q10,” “CoQ10,” “ubiquinone,” “diabetic nephropathy,” “diabetic kidney disease,” “western medicine,” “placebo,” “clinical trials,” and “experimental study.” The free-text and Medical Subject Headings (MeSH) term searching strategy was conducted to ensure the objective and comprehensive aim.
The language of searching term mention above was slightly adjusted in Chinese medical databases (CBM, CNKI, CMCI, VIP, and Wanfang database) based on the translation adaptation. The method of hand searching was performed in the library of the Beijing University of Chinese Medicine to identify whether the potential articles existed. The flow diagram was conducted by the software RevMan (Review Manager, version 5.3, the Nordic Cochrane Center, the Cochrane Collaboration, 2012 Copenhagen, Denmark), illustrating the identification, inclusion, and exclusion of articles (Fig. 1).
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
2.3. Study selection
The selection of studies was performed by two reviewers’ cooperation (ZS and QL) and verified by the third reviewer (XZ) through the software Endnote (version X8, Clarivate Analytics, 2017 Boston, MA), ensuring the objectiveness and quality of process.
Appropriate literatures were included in the meta-analysis if they suited the following criteria: full-text accessible clinical trials (randomized controlled trial, cohort studies, and case-control studies) and experimental studies; patients were diagnosed with type1 and type 2 DKD without age or sex restriction; the type1 or type 2 DKD animal model was well established (i.e., the Wistar rats injected by streptozotocin [STZ][28] or the BKS.Cg-Dock7m+/+Leprdb/J [db/db] mice[29]); experimental group: patients or animal were received CoQ10 combined with related western medicines or placebo, without consistent dose requirement; control group: participants or animal received placebo or western medicines; the type of outcome measures for patients should include at least one aspect as followed: fasting plasma glucose (FPG, mmol/L); glycated hemoglobin A1c (HbA1c, %); low density lipoprotein cholesterol (LDL-C, mmol/L); high density lipoprotein cholesterol (HDL-C, mmol/L); total cholesterol (TC, mmol/L); triglyceride (TG, mmol/L); GFR (g) Urea (mmol/L)/blood urea nitrogen (BUN, mmol/L), serum creatinine (Scr, μmol/L), Cystatin C (Cys-C, mg/L); β2-microglobulin (β2-MG, mg/L); malondialdehyde (MDA, μmol/L). The outcome measures for animal model should contain one aspect suit the following: glucose (mmol/L); urine output (ml/24 h).
Articles were excluded from the meta-analysis if they do not meet the criteria mentioned before or meet conditions as followed: non type 1 or type 2 DKD patients (i.e., gestational diabetes) or animal model; severe clinical illnesses (such as acute kidney disease) or infections; the final stage DKD or ESRD (GFR < 15 mL/min or requirement for the operation of renal replacement); inconsistency of methods and outcomes; data missing; not setting experimental or control group; reviews, editorials, letters, meta-analysis, comments and so forth.
2.4. Data extraction
The data of studies were extracted by 2 independent reviewers (ZS and QL) based on inclusion and exclusion criteria. Any possible discrepancies were judged and discussed by the third reviewer (XZ) until the final conclusion was made.
The extracted data of included articles contained as followed: the author's name and the year of publication; the type of disease; nationality and race (including the number of men and women); sample size (experimental group/controlled group); the age of included participants/animal model; study design; random methods; treating method of intervention group; treating method of controlled group; the duration of intervention; major outcome. However, it should be highlighted that the units of evaluating index were unified through the way of calculating conversion.[30] The authors of included articles were contacted if the literature characteristics cannot be obtained (Table 1 ).
Table 1.
General characteristics of included studies.

2.5. Quality analysis
The study quality analysis was conducted by 2 investigators (ZS and HQ) based on the Cochrane Collaboration's tools for the risk of bias assessment.[31] This tool contained 6 domains for the literature's evaluation (only for randomized controlled trials [RCTs][37–40]): the random allocation method; the allocation concealment; the blinding method; the outcome data integrity; the outcome data of selective reports; other bias sources.
The evaluating criteria of study sheet defined as “Low risk,” “High risk,” and “Unclear risk” was conducted. If an included study had 3 or more “Low risk,” this study should be recognized as being of high quality which has low risk of bias, and vice versa. The literature data were collected and analyzed efficiently by the software Review Manager (RevMan; version 5.3; the Nordic Cochrane Center, the Cochrane Collaboration, 2012 Copenhagen, Denmark) and presented at table (Table 2) and figure (Figs. 2 and 3).
Table 1 (Continued).
General characteristics of included studies.

Figure 2.

Risk of bias graph: judgements of reviewers about each risk of bias item presented as percentages across all included RCT studies. RCT = randomized controlled trials.
Figure 3.

Risk of bias summary: judgements of reviewers about each risk of bias item for each included RCT study. RCT = randomized controlled trials.
Table 2.
The risk bias evaluation of articles (RCTs).

2.6. Statistical analysis
Data of articles were pooled and analyzed by the software RevMan. The risk ratio (RR) with 95% confidence interval (CI) for dichotomous outcomes and the standard mean difference (SMD) or weighted mean difference (WMD) with 95%CI for continuous outcomes was calculated, respectively, by the author ZS and repeating verified by the author XZ.
The I2 statistical calculation was applied for the heterogeneity for the purpose of explanation of potential inconsistency across the included studies. This statistic, which is a quantitative tool, indicating the degree of heterogeneity by the percentage of the article's variation as a percentage of the total variation: it would be recognized as being of low heterogeneity when the result of I2 statistic is between 25% and 50%; the result between 50% and 75% would be of moderate heterogeneity, and >75% would be of high heterogeneity. The result of I2 statistic was regarded as obtaining the heterogeneity when it exceeding 50% according to the Cochrane Handbook (version 5.1.0) notation.[32] For studies with significant heterogeneity, heterogeneity testing needs to be performed according to the number of studies. The major method is to conduct the meta-regression analysis of single covariates and multiple covariates in the software named Stata (version 14; StataCorp, 4905 Lakeway Drive, College Station, TX) to explore the source of heterogeneity. However, the heterogeneity cannot be fully explained through this way and the residual heterogeneity was allowed for existence. The fixed-effects model (the Mantel-Haenszel method for dichotomous variation and the Inverse-Variance method for continuous variation) was performed to analyze data if the heterogeneity did not exist or was moderate.[33] The random effects model applying the Der Simonian-Laired method were conducted if the heterogeneity was high.[34]
The sensitivity analysis was adopted to explore the stability of included studies. The publication bias was evaluated by a funnel plot, Egger test, and Begg test based on the number of studies through the software Stata. The asymmetry of image in visual appearance or P value <.05 in Egger test or Begg test calculation could be recognized as having the publication bias.[35,36]
3. Results
3.1. Search results
The flowchart based on the PRISMA was presented on Fig. 1.
A total of 178 potentially relevant citations were initially identified according to the search strategies from 12 electronic medical databases. Thirty-four duplicated literatures were excluded and further 144 records were performed next-step evaluation. After screening the titles and abstracts, 105 records were excluded for the following reasons: 21 studies were reviews; 8 records were letters; 11 studies were conference papers; 2 literatures were editorials; 26 studies were meta-analyses; 5 records were comments; 32 studies were analyzed irrelevant diseases. Thirty-nine full-text articles were assessed for eligibility and 29 of them were excluded for the following reasons: 6 studies could not obtain full-text papers even E-mail the authors; 5 articles missed the experimental or control groups; 9 studies contained CoQ10 in controlled group; 7 studies were clinical protocols; 4 studies missed the data of results. Finally, a total of 8 articles (4 clinical trials and 4 experimental studies) were enrolled in this meta-analysis.[37–44] (Table 1 )
3.2. Study general characteristics
The details and characteristics of included 8 articles (4 RCTs with 175 patients and 4 experimental studies with 58 rats) were clearly illustrated in Table 1 (designed and made by XZ). The clinical and experimental articles were grouped based on the type of disease, nationality or races, sample size, age, study design, random method, the intervention method of experimental and controlled group, duration and major methods.
The published year of articles was between 2011 and 2018. All included articles selected type 1 or 2 DKD as target for experimental or clinical study. RCTs were all conducted in Asia (1 in China[38] and 2 in Iran[39,40]) except 1 in Russia.[37] The numbers of male and female of RCTs were distinctly shown in 2 studies.[37,38] As for included experimental studies, the category of the animal model can be divided as 2: db/db mice with DKD[41,44] and Wistar rats with DKD.[42,43] The sample size varied from 20 to 65 for RCTs and 12 to 20 for experimental studies. The age varied from 18 to 85 years old for participants of RCTs and 8 to 12 weeks for animal models. The intervention design of DKD for clinical evaluation, 2 of which applied CoQ10 in combination with western medicine (insulin[37] and atorvastatin[38]) versus western medicine alone, and the other 2 selected CoQ10 alone versus placebo.[39,40] As for experimental studies, 2 of which performed CoQ10 in combination with western medicine (metformin[43] and sitagliptin[44]) versus western medicine alone, and the other 2 selected CoQ10 alone versus distilled water. The treating duration of RCTs was unified for 12 weeks expect 1 literature.[37] The duration of experimental studies was varied from 2 to 10 weeks. The major outcomes for included RCTs and experimental studies were clearly presented on Table 1 and verified by 2 authors’ cooperation (QL and HQ). As for the data, which did not show on the published articles, reviewers of meta-analysis (XZ) had tried to contact the authors by E-mail but did not obtain useful information.
3.3. Quality evaluation
The quality evaluation of included RCTs was based on the Cochrane Collaboration tools for the risk of bias assessment was presented on Table 2. Two RCTs were provided by the “Random sequence generation (selection bias)” method,[39,40] however, no articles showed the way of “Allocation concealment (selection bias).” Two RCTs were presented by the method of “Blinding of participants and personnel (performance bias)”.[39,40] Only one RCTs clearly illustrated and certain the method of “Blinding of outcome assessment (detection bias).”[39] All included RCTs had no attrition bias except one,[38] which could not access the integrated information from the article. All outcome data of included trials were integrity and could achieve the expected outcome without the reporting bias. Only one included article did not conform to the outcome data of selective report because the outcomes were merely presented by value without specific outcome data supporting.[39] For the part of the “Other bias,” only one article had not enough information to evaluate whether there is an important risk of bias.[38]Figures 2 and 3 were conducted by the software RevMan by 2 reviewers’ (ZS and QL) cooperation and made the Table 2 on the next step. Disagreements among this process were discussed with the third reviewer (XZ) until the same final conclusion was made.
3.4. Meta-analysis /systematic review
3.4.1. The clinical indexes of DM (FPG and HbA1c) for RCTs
Figures 4 and 5 clearly showed the variation of clinical indexes (fasting plasma glucose, mmol/L; glycated hemoglobin, %) in DKD therapy.
Figure 4.

A, The forest plot of FPG (fasting plasma glucose, mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of FPG (mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
Figure 5.
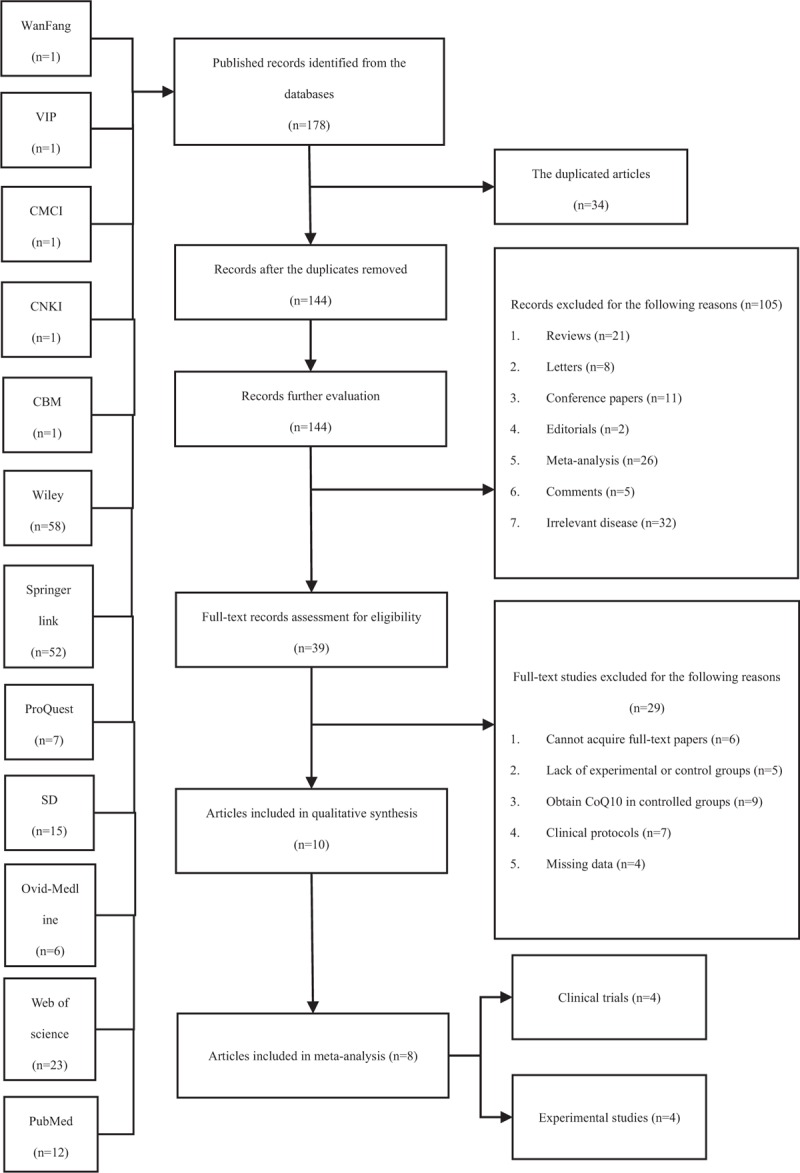
A, The forest plot of HbA1c (glycated hemoglobin, %) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of HbA1c level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
In a pooled meta-analysis of fasting plasma glucose (FPG) in forest plot (Fig. 4A),[37–39] 3 studies containing 135 patients were included. For the 3 articles, 1 applied CoQ10 in combination with insulin[37] in the experimental group while one used CoQ10 combined with atorvastatin[38] and one chose CoQ10 alone.[39] Std. MD was conducted as a combined statistic for the reason of the obvious difference of standard deviation (SD) of the results. The heterogeneity of FPG was extremely high (I2 = 93%), so the random effects model was performed to calculate the combined data by inverse variance (IV) test. We conducted the subgroup analysis (Fig. 4B) by the software Stata based on the methods of treatment (combined therapy or single therapy), the test area (Asia or Europe), the sample size, and the quality of study (high or low) to analyze the source of heterogeneity. The result showed the source of heterogeneity may come from the methods of treatment (I2 = 0) and the quality of study (I2 = 0). The meta-analysis illustrated that the FPG level for CoQ10 combined with western medicine after DKD treatment was superior to placebo or western medicine (Std. MD = –2.04, 95% CI = –3.90 to –0.18, and P < .05). The result had statistical difference (Fig. 4A).
Figure 5A was the forest plot of HbA1c level after CoQ10 therapy for experimental groups and western medicine or placebo treatments for control groups. Three appropriate articles including 135 patients were collected and analyzed in meta-analysis[37–39] and the Std. MD was conducted as a combined statistic. We found that the heterogeneity of included studies was obvious (I2 = 94%), so the random effects model was applied by IV approach. The result of subgroup analysis (Fig. 5B) demonstrated that the source of heterogeneity may come from the quality of included articles (I2 = 0). The meta-analysis showed that the HbA1c level of the experimental group did not show the superiority compared with the control group (Std.MD = −1.83, 95% CI = −3.39 to −0.27, and P > .05) and had no statistical difference (Fig. 5A).
3.4.2. The clinical indexes of blood lipid (TC, HDL-C, LDL-C, and TG) for RCTs
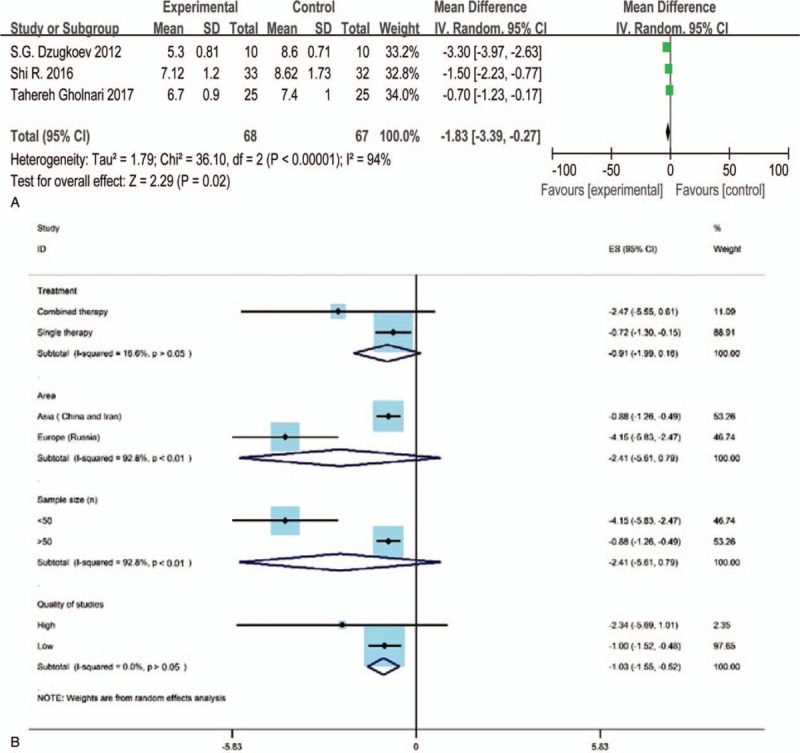
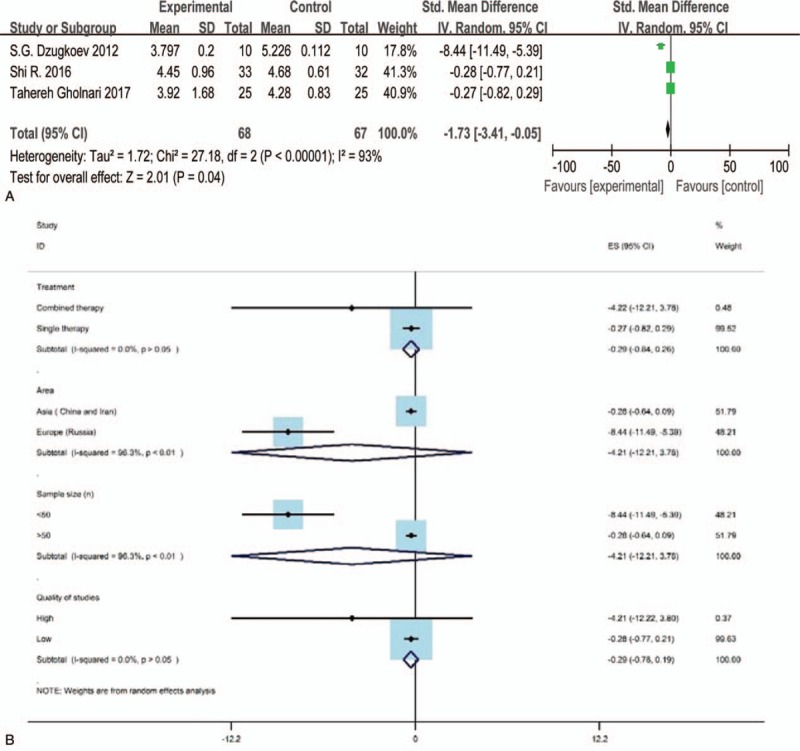
Three studies[37–39] evaluated the result of TC, HDL-C, LDL-C after CoQ10 treatment for experimental groups and western medicine or placebo treatments for control groups, respectively. Only 2 studies reported the result of TG.[38,39] The Std. MD was conducted as a combined statistic. The random effects model was performed in the clinical indexes evaluation of TC (I2 = 93%) and HDL-C (I2 = 57%) for the reason of obvious heterogeneity, while the fixed effects model was conducted in the indexes evaluation of LDL-C and TG based on the low heterogeneity (I2 = 0%). The result of subgroup analysis showed that the article's quality (I2 = 0) is the source of heterogeneity for TC and HDL-C, however, the methods of treatment (I2 = 0) only contributed to the source of heterogeneity for TC (Figs. 6B and 8B). The meta-analysis (Figs. 6A, 8A, and 9) illustrated that the level of TC, HDL-C and TG in the experimental group was spurious to the control group after therapy (TC: Std.MD = −1.73, 95% CI = −3.41 to –0.05, and P < .05; HDL-C: Std. MD = 0.09, 95% CI = 0.01–0.18, and P < .05; TG: Std.MD = −0.39, 95% CI = −0.71 to –0.07, and P < .05), demonstrating the statistical difference of results. On the other hand, the level of LDL-C (Fig. 7) had no statistical difference (Std.MD = –0.27, 95% CI = –0.62 to 0.07, and P > .05).
Figure 6.
A, The forest plot of TC (total cholesterol, mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of TC level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
Figure 8.

A, The forest plot of HDL-C (high-density lipoprotein cholesterol, mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of HDL-C level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
Figure 9.

The forest plot of TG (triglyceride, mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
Figure 7.
The forest plot of LDL-C (low-density lipoprotein cholesterol, mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
3.4.3. The clinical indexes of renal function for RCTs
Seven kinds of renal function indicators (GFR, Proteinuria, Creatinine, Serum Creatinine, CysC, β2-MG, and Urea) were evaluated in the 4 included RCTs,[37–40] one of which included a meta-analysis (Urea).
Only one RCTs[37] evaluated the indexes of GFR, Proteinuria, and Creatinine for renal function of DKD. The outcomes indicated that the efficacy of the combined group (Insulin + CoQ10) superior to the single therapy group (Insulin) with statistical difference (P < .001). The evaluation of serum creatinine in a RCTs[39] showed that there was no statistical difference (P > .01) between the CoQ10 group and the placebo group. As for the indexes of CysC and β2-MG for renal function, only one included RCTs[38] assessed them. The results indicated that the combined group (Atorvastatin + CoQ10) contained statistical difference compared with the control group (Atorvastatin) for β2-MG (P < .05). However, the CysC indicator had no statistical difference (P > .05).
The forest plot of Urea level after CoQ10 treatment for experimental groups and western medicine or placebo treatments for control groups was illustrated in the Fig. 10A. Two appropriate articles including 70 patients were collected and analyzed in meta-analysis[37,39] and the Std. MD was conducted as a combined statistic. We found that the heterogeneity of included studies was obvious (I2 = 94%), so the random effects model was applied by IV approach. The result of subgroup analysis (Fig. 10B) did not find the source of heterogeneity. The meta-analysis showed that the Urea level of the experimental group was not superior to the control group (Std.MD = −1.24, 95% CI = −4.04 to 1.55, and P > .05) and had no statistical difference (Fig. 10B).
Figure 10.

A, The forest plot of urea (mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of urea level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, RCT = randomized controlled trials.
3.4.4. The oxidative injury index (MDA) for RCTs
Two studies of 115 patients were included and evaluated the index of oxidative damage named MDA between CoQ10 for experimental groups and western medicine or placebo treatments for control groups in Fig. 11A. The Std. MD was conducted as a combined statistic. The random effects model was applied by IV approach based on the high heterogeneity (I2 = 84%). The source of heterogeneity was not found after the subgroup analysis (Fig. 11B). The result of meta-analysis illustrated that the MDA level of the experimental group was superior to the control group (Std.MD = −1.29, 95% CI = −2.32 to –0.26, and P < .05) and had the statistical difference.
Figure 11.

A, The forest plot of MDA (μmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. B, Subgroup analysis of MDA level for CoQ10 intervention versus placebo or western medicine after DKD treatment in RCTs. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance, MDA = malondialdehyde, RCT = randomized controlled trials.
3.4.5. The parameters of DKD for experimental studies (glucose and urine output)
There were 2 experimental indicators included in meta-analysis based on the number of included rats for experimental studies.
Figure 12 was the forest plot of glucose level (mmol/L) after CoQ10 intervention for experimental groups and distilled water for control groups. Two appropriate articles including 34 db/db mice were collected and analyzed in meta-analysis[41,44] and the Std. MD was applied as a combined statistic. The fixed effects model was performed by IV approach for reason of low level of heterogeneity (I2 = 2%). However, the result showed that the biochemical parameter of glucose level for the experimental group was not superior to the control group (Std.MD = −0.03, 95% CI = −0.65 to 0.71, and P > .05) and had no statistical difference (Fig. 12).
Figure 12.
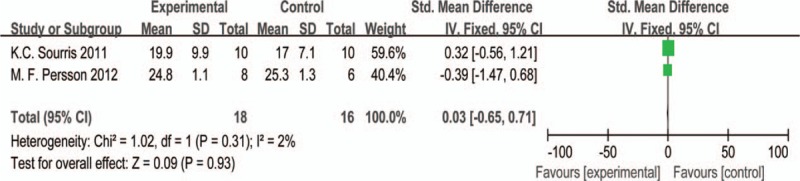
The forest plot of glucose (mmol/L) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in experimental studies. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance.
The parameter of urine output evaluation was analyzed in meta-analysis based on CoQ10 intervention for experimental groups compared with distilled water for control groups.[41,44] The Std. MD was conducted as a combined statistic and the random effects model was performed by IV approach for reason of the high level of heterogeneity (I2 = 70%). Although the subgroup analysis (area, published time, and sample size) was conducted by the software Stata, the results did not reveal the precise source of heterogeneity (Fig. 13B). The meta-analysis illustrated that the urine output level of the experimental group did not show the superiority compared with the control group (Std.MD = −0.23, 95% CI = −1.54 to 1.08, and P > .05) and had no statistical difference (Fig. 13A).
Figure 13.

A, The forest plot of urine output (mL/24 h) level for CoQ10 intervention versus placebo or western medicine after DKD treatment in experimental studies. B, Subgroup analysis of urine output level for CoQ10 intervention versus distilled water for DKD in experimental studies. CI = confidence interval, DKD = diabetic kidney disease, IV = inverse variance.
3.5. The analysis of publication bias
The funnel plot (Fig. 14) was drawn by the reviewer (ZS) based on pooled odds ratio (OR) as the midpoint to analyze the publication bias. The publication bias of major parameters for included RCTs was evaluated by comparing the symmetry of the funnel plot.
Figure 14.
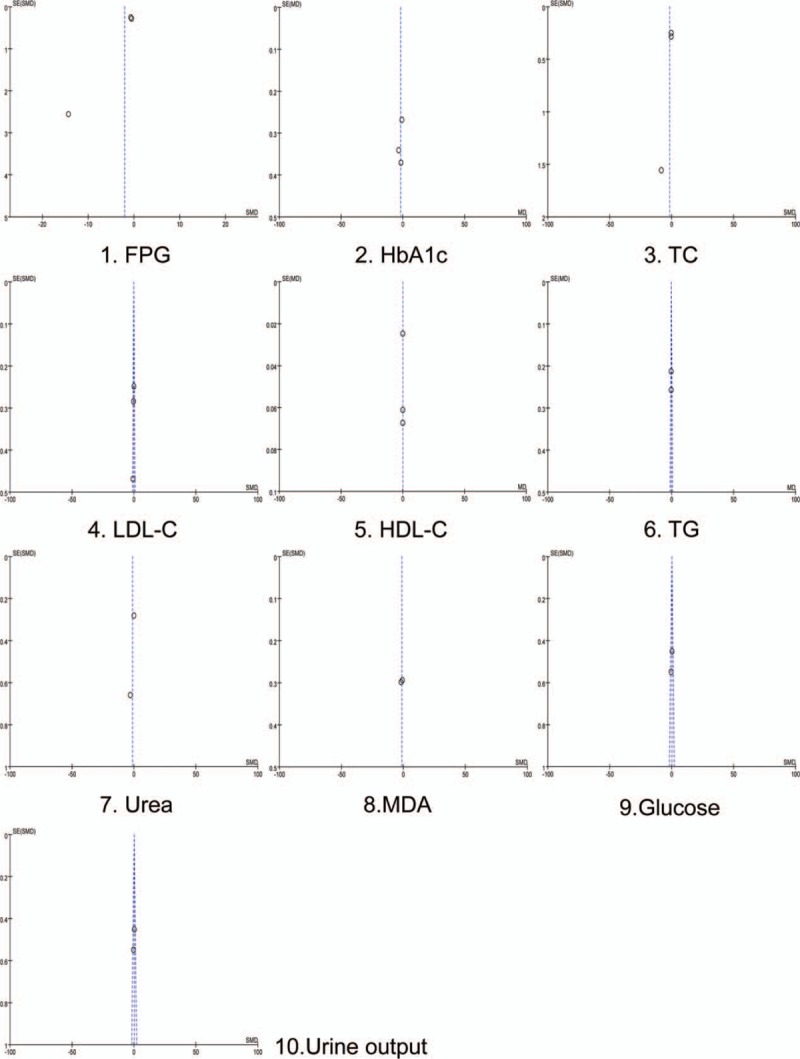
The funnel plot of major parameters for included RCTs.
The symmetry of funnel plot was assessed by 2 reviewers (QL and HQ) in the visual point and both the reviewers considered that these images were symmetrical except one (FPG index), which means that all parameters except one did not have the publication bias. We performed the Egger test and Begg test to further confirm whether the publication bias for FPG index did exist (Fig. 15). The result of Egger (t = –5.75, P > .05) and Begg test (z = 0, P > .05) and images were made by Stata, indicating that the publication bias did not exist.[36] However, it is needed to highlight that the limited numbers of RCTs for clinical parameters restricted this application.
Figure 15.
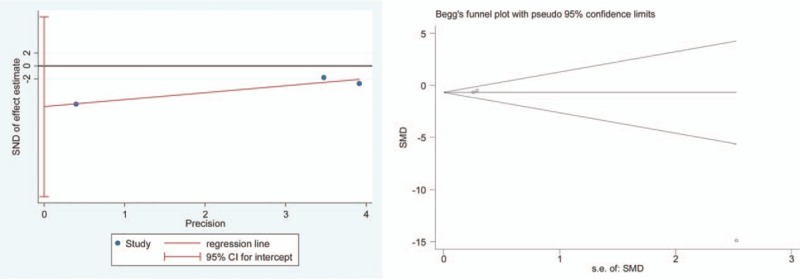
Egger's test and Begg's test for the index of FPG. FPG = fasting plasma glucose.
3.6. The sensitive analysis
The different effects model was performed to conduct the sensitive analysis. This procession was conducted in the software RevMan (version 5.3). If the statistical heterogeneity did exist between the included studies and the sample size is small, it indicates that the data of small sample study will affect the overall combined effect of meta-analysis.
The RevMan was applied to analysis the results of clinical indexes with high heterogeneity (FPG, HbA1c, TC, HDL-C, urea, MDA, urine output) for meta-analysis through the method of transforming the fixed effect model to the random effect model and vice versa. If the results are similar, the small sample data with high heterogeneity do not significantly affect the overall combined effect in meta-analysis. The sensitive results illustrated that the stability was obtained in our meta-analysis.
4. Discussion
4.1. The recommendation of CoQ10 for DKD
DKD is mainly caused by the disease of DM and is the primary cause of kidney disease for people (20–40%) who starting treatment for ESRD worldwide.[45] Reduced the progression of kidney damage and controlled the related complications are the goal of DKD therapy. The major method is the ACE inhibitor medications when the proteinuria was appeared, which can obviously slow the progression of DKD.[46] The control and management of high blood pressure and blood sugar levels are also essential for DKD treatment. The theory of the relationship between CoQ10 and renal disease was built up with the fund of a complicated syndrome characterized by steroid resistant nephrotic syndrome (SRNS).[47] Several articles indicated that antioxidant supplements, the CoQ10 for example, may have potential benefits to DKD therapy.[48] However, it should be noted that whether CoQ10 need be systematically recommended for retarding DKD progression still in the controversy and lack of evidence-based medicine support.
4.2. Summary of the major evidence
This systematic review and meta-analysis have shown the clinical effectiveness of CoQ10 in RCTs and the amelioration of related indexes in experimental studies for DKD therapy by collecting 8 high quality articles from 12 electronic medical databases. The evidence-based rules of CoQ10 in combination with western medicine for DKD have been conducted by this systematic review and meta-analysis.
There are 4 obvious advantages for our study: the included articles basically have good quality based on the Cochrane Collaboration tools for the risk of bias assessment, ensuring the results trustworthy; the quality of included studies was evaluated by 3 reviewers’ cooperation (XZ, ZS, and QL); the source of heterogeneity was explored by the method of the subgroup analysis; the publication bias of included articles was analyzed by the funnel plot, the Egger test and Begg test; the author was contacted by our reviewers through the way of E-mail if an appropriate study cannot be acquired as full-text.
The results of the FPG, the Glycated HbA1c, TC, HDL-C, TG, and MDA in meta-analysis illustrated that the experimental group (CoQ10 combined with western medicine or not) was superior to the control group (placebo or western medicine) after DKD treatment (P < .05). The subgroup analysis revealed that the source of heterogeneity may come from the different way of treatment for the parameters of FPG and TC and the quality of articles for the indexes of HbA1c, TC, and HDL-C (P = 0%). However, the subgroup analysis did not reveal the source of heterogeneity for the parameter of MDA. The result of the low-density lipoprotein cholesterol (LDL-C) and urea level in meta-analysis showed that the experimental group was irrelevant to the control group after DKD therapy (P > .05). The subgroup analysis did not reveal the source of heterogeneity for the index of Urea level. The meta-analysis of experimental articles indicated that the results of glucose and urine output in the experimental group (CoQ10) were irrelevant to the control group (distilled water) and the subgroup analysis did not reveal the source of heterogeneity. The outcomes of Egger test, Begg test, and funnel plot did not reveal the publication bias and the sensitive analysis proved the stability of our study.
4.3. Limitations
This systematic review and meta-analysis had several potential limitations restricting the clinical application of CoQ10 for DKD: included studies are smaller (4 RCTs and 4 experimental studies) than we expected although all obtained the high quality; the number of participants or animal models for experimental group and control group in included studies is small; the heterogeneity of some included studies is obvious although reviewers conducted study selection, data extraction, and quality analysis strictly based on the outline.[27] Subgroup analysis for some indexes (urea level and urine output) also could not clearly reveal the source of heterogeneity. We need to understand that the random effects model which performed to pool data cannot give exact and stable conclusion in this condition; the combined therapy for CoQ10 are diversified in the included RCTs: some studies used western medicines (insulin or atorvastatin) and some studies did not combine. Also, therapy in the control group could not reach a consensus as a uniform method (insulin or atorvastatin or placebo).
5. Conclusion
In conclusion, this systematic review and meta-analysis provided the practical and beneficial results of CoQ10 therapy for DKD to some extent. Although the indexes of FPG, HbA1c, TC, HDL-C, TG, and MDA amelioration after DKD therapy with CoQ10 combined with western medicine in the meta-analysis showed a statistical difference, the clinical meaning was restricted by the shortage of included articles. So this aspect conclusion needs to be confirmed by further clinical research in the future. As for evaluating the indexes of LDL-C and urea level for RCTs and urine output and glucose for experimental studies, we did not find meaningful outcome for the superiority of CoQ10 treatment in DKD. It should be noticed that further and deeper standard, multicenter, double-blind RCTs, and formal experimental studies of CoQ10 treatment for DKD were urgent to be conducted for more clinical evidence providing in the future. The underlying pharmacological mechanism of CoQ10 needs to be researched and revealed for its future application of DKD therapy.
Author contributions
Xiaofeng Zhang and Zhaofeng Shi have contributed equally to this work. Xiaohong Cheng is the corresponding author of this article. The databases were searched and selected by Xiaofeng Zhang, Zhaofeng Shi, and Xiaohong Cheng. The data of included articles were extracted and performed the quality analysis by Xiaofeng Zhang. The statistical analysis was conducted by Xiaofeng Zhang and Zhaofeng Shi. Study tables and figures were conducted by Zhaofeng Shi and Qian Liu. The basic knowledge of western medicine application and evidence-based medical ways was guided by Qian Liu and Haohao Quan, respectively.
Conceptualization: Xiaofeng Zhang.
Data curation: Xiaofeng Zhang.
Formal analysis: Xiaofeng Zhang.
Investigation: Haohao Quan.
Methodology: Haohao Quan.
Project administration: Xiaohong Cheng.
Resources: Haohao Quan.
Software: Qian Liu.
Supervision: Zhaofeng Shi.
Writing – original draft: Zhaofeng Shi.
Footnotes
Abbreviations: β2-MG = β2-microglobulin, BUN = blood urea nitrogen, CBM = Chinese BioMedical Literature Database, CI = confidence interval, CKD = chronic kidney disease, CMCI = Chinese medical Citation Index, CNKI = China National Knowledge Infrastructure, CoQ10 = Coenzyme Q10, CVD = cardiovascular diseases, CysC = Cystatin C, DKD = diabetic kidney disease, DM = diabetes mellitus, DR = diabetic retinopathy, ESRD = end-stage renal disease, FPG = fasting plasma glucose, GBM = glomerular basement membrane, HbA1c = Hemoglobin A1c, HDL-C = high density lipoprotein cholesterol, IL-1 = Interleukin-1, IV = inverse variance, LDL-C = low density lipoprotein cholesterol, MDA = malondialdehyde, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RAS = Renin-Angiotensin System, RCTs = randomized controlled trials, ROS = reactive oxygen species, RR = risk ratio, Scr = serum creatinine, SD = standard deviation, SGLT2 = sodium glucose cotransporter 2, SMD = standard mean difference, SRNS = steroid resistant nephrotic syndrome, Std. MD = standard mean difference, STZ = streptozotocin, TC = total cholesterol, TG = triglyceride, TGF-β = transforming growth factor-β, TNF-α = tumor necrosis factor-α, UAE = urinary albumin excretion, VIP = Chinese Scientific Journal Database, WMD = weighted mean difference.
XZ and ZS have contributed equally to this work.
This systematic review and meta-analysis was supported by the Science Fund Project of China National Natural Science Foundation (No. 81873201, No. 81503441).
All contributing authors have declared that there are no conflicts of interest.
References
- [1].Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 2000;341:1127. [DOI] [PubMed] [Google Scholar]
- [2].Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Umanath K, Lewis JB. Update on diabetic nephropathy: core Curriculum 2018. Am J Kidney Dis 2018;71:884–95. [DOI] [PubMed] [Google Scholar]
- [5].Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. J Am Med Assoc 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- [6].Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med 2016;375:905–6. [DOI] [PubMed] [Google Scholar]
- [7].Foundation NK. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kid Dis 2007;492 suppl 2:S12–54. [DOI] [PubMed] [Google Scholar]
- [8].Reutens AT, Atkins RC. Epidemiology of diabetic nephropathy. Contrib Nephrol 2011;170:1–7. [DOI] [PubMed] [Google Scholar]
- [9].Li J, Liang TT, Wang WJ. The early diagnosis of diabetic kidney disease. Chin J Nephrol 2017;33:470–2. [Google Scholar]
- [10].Tervaert TWC, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556–63. [DOI] [PubMed] [Google Scholar]
- [11].Ponchiardi C, Mauer M, Najafian B. Temporal profile of diabetic nephropathy pathologic changes. Curr Diab Rep 2013;13:592–9. [DOI] [PubMed] [Google Scholar]
- [12].Prospective Diabetes Study (UKPDS) Group UK. “Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 Diabetes (UKPDS 33)”. Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- [13].Duckworth W, Abraira C, Moritz T, et al. “Glucose control and vascular complications in veterans with type 2 diabetes”. N Engl J Med 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- [14].Griffin SJ, Borchjohnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomized trial. Lancet 2011;378:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol 2014;25:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lewis EJ, Hunsicker LG, Clarke WR, et al. Reno protective effect of the Angiotensin-Receptor Antagonist Irbesartan in patients with nephropathy due to Type 2 diabetes. N Engl J Med 2001;345:851–60. [DOI] [PubMed] [Google Scholar]
- [17].Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–903. [DOI] [PubMed] [Google Scholar]
- [18].Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a Randomized Clinical Trial. JAMA 2015;314:884–94. [DOI] [PubMed] [Google Scholar]
- [19].Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation 2016;134:752–72. [DOI] [PubMed] [Google Scholar]
- [20].Ozaltin F. Primary coenzyme Q10 (CoQ10) deficiencies and related nephropathies. Pediatr Nephrol 2014;29:961–9. [DOI] [PubMed] [Google Scholar]
- [21].Ayers J, Cook J, Koenig RA, et al. Recent developments in the role of Coenzyme Q10 for coronary heart disease: a systematic review. Curr Atheroscler Rep 2018;20:29. [DOI] [PubMed] [Google Scholar]
- [22].Flowers N, Hartley L, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;CD010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moradi M, Haghighatdoost F, Feizi A, et al. Effect of Coenzyme Q10 supplementation on diabetes biomarkers: a systematic review and meta-analysis of randomized controlled clinical trials. Arch Iran Med 2016;19:588–96. [PubMed] [Google Scholar]
- [24].Pirro M, Mannarino MR, Bianconi V, et al. The effects of a nutraceutical combination on plasma lipids and glucose: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2016;110:76–88. [DOI] [PubMed] [Google Scholar]
- [25].Ishikawa A, Kawarazaki H, Ando K, et al. Renal preservation effect of Ubiquinol, the reduced form of Coenzyme Q10. Clin Exp Nephrol 2011;15:30–3. [DOI] [PubMed] [Google Scholar]
- [26].Bolignano D, Cernaro V, Gembillo G, et al. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis. PLos One 2017;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- [28].Johansen NJ, Tripovic D, Brock JA. Streptozotocin-induced diabetes differentially affects sympathetic innervation and control of plantar metatarsal and mesenteric arteries in the rat. Am J Physiol Heart Circ Physiol 2013;304:H215–28. [DOI] [PubMed] [Google Scholar]
- [29].Iii FCB, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol 2005;16:27–45. [DOI] [PubMed] [Google Scholar]
- [30].People's Military Medical Publishing House, Chinese Medical Association Magazine. The Application of Legal Measurement Units in Medicine, (the Third Edition). 1987. [Google Scholar]
- [31].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org. [Google Scholar]
- [32].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Donald A, Donner A. Adjustments to the mantel-haenszel chi-square statistic and odds ratio variance estimator when the data are clustered. Stat Med 1987;6:491–9. [DOI] [PubMed] [Google Scholar]
- [34].Böhning D, Malzahn U, Dietz E, et al. Some general points in estimating heterogeneity variance with the DerSimonian-Laird Estimator. Biostatistics 2002;3:445–57. [DOI] [PubMed] [Google Scholar]
- [35].Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- [36].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1998;316:469–71. [Google Scholar]
- [37].Dzugkoev SG, Dzugkoeva FS. Effect of combination therapy with coenzyme Q10 on functional and metabolic parameters in patients with Type 1 Diabetes Mellitus. Bull Exp Biol Med 2012;152:364–6. [DOI] [PubMed] [Google Scholar]
- [38].Shi R, Li JD, Feng F, et al. Effects of atorvastatin combined with coenzyme Q10 on elderly Type 2 diabetic nephropathy. Chin J Gerontol 2016;36:5875–6. [Google Scholar]
- [39].Gholnari T, Aghadavod E, Soleimani A, et al. The effects of Coenzyme Q10 supplementation on glucose metabolism, lipid profiles, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr 2017;37:188–93. [DOI] [PubMed] [Google Scholar]
- [40].Heidari A, Hamidi G, Soleimani A, et al. Effects of Coenzyme Q10 supplementation on gene expressions related to insulin, lipid, and inflammation pathways in patients with diabetic nephropathy. Iran J Kidney Dis 2018;12:14–21. [PubMed] [Google Scholar]
- [41].Sourris KC, Harcourt BE, Tang PH, et al. Ubiquinone (Coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of Type 2 diabetes. Free Radic Biol Med 2012;52:716–23. [DOI] [PubMed] [Google Scholar]
- [42].Persson MF, Catrina SB, Dallner G, et al. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyper filtration and proteinuria in kidneys from db/db mice as a Model of Type 2 diabetes. Diabetologia 2012;55:1535–43. [DOI] [PubMed] [Google Scholar]
- [43].Maheshwari RA, Balaraman R, Sen AK, et al. Effect of Coenzyme Q10 alone and its combination with metformin on streptozotocin-nicotinamide-induced diabetic nephropathy in rats. Ind J Pharmacol 2014;46:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maheshwari R, Balaraman R, Sen AK, et al. Effect of Concomitant administration of Coenzyme Q10 with sitagliptin on experimentally induced diabetic nephropathy in rats. Ren Fail 2017;39:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, “International comparisons. In: 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States,” Bethesda: NIH and NIDDKD, 2007: 239–254. [Google Scholar]
- [46].Lim AK. Diabetic nephropathy-complications and treatment. Int J Nephrol Renovasc Dis 2014;7:361–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rötig A, Appelkvist EL, Geromel V, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 2000;356:391–5. [DOI] [PubMed] [Google Scholar]
- [48].Bhattacharjee N, Barma S, Konwar N, et al. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol 2016;791:8–24. [DOI] [PubMed] [Google Scholar]


