Figure 1.
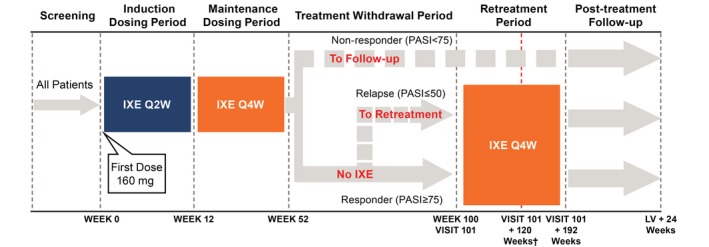
Study design (patients with plaque psoriasis). All patients received 160‐mg doses of ixekizumab at Week 0 (Visit 2; all doses during the study were delivered by SC injection) and 80 mg IXE Q2W from Week 2 (Visit 4) to Week 12 (Induction Dosing Period). During the Maintenance Dosing Period and Retreatment Period, patients received 80 mg IXE Q4W. At Week 52, patients with plaque psoriasis were classified as a responder (PASI75) or non‐responder (PASI <75). Non‐responders entered the Post‐treatment Follow‐up Period. Responders moved to the Treatment Withdrawal Period. Patients who completed the Treatment Withdrawal Period (Week 100) entered the Retreatment Period. If a patient experienced a relapse (loss of response; PASI ≤50) at any visit prior to Week 100, the patient moved to the Retreatment Period with a duration of 192 weeks. †Nine months after marketing authorization was received in Japan, all patients stopped administration of ixekizumab and moved to post‐treatment follow‐up. Patients who had completed the Retreatment Period or were ongoing were counted as having completed the Retreatment Period. At the Retreatment Period cut‐off date (3 April 2017), all completed patients had at least 120 weeks of retreatment. IXE, ixekizumab; IXE Q2W, 80 mg ixekizumab every 2 weeks; IXE Q4W, 80 mg ixekizumab every 4 weeks; LV, last regularly scheduled visit or early termination visit; PASI50/75, ≥50%/≥75% improvement in the Psoriasis Area Severity Index; SC, subcutaneous.
