Abstract
Hidradenitis suppurativa (HS)/acne inversa is a debilitating chronic disease that remains poorly understood and difficult to manage. Clinical practice is variable, and there is a need for international, evidence‐based and easily applicable consensus on HS management. We report here the findings of a systematic literature review, which were subsequently used as a basis for the development of international consensus recommendations for the management of patients with HS. A systematic literature review was performed for each of nine clinical questions in HS (defined by an expert steering committee), covering comorbidity assessment, therapy (medical, surgical and combinations) and response to treatment. Included articles underwent data extraction and were graded according to the Oxford Centre for Evidence‐based Medicine criteria. Evidence‐based recommendations were then drafted, refined and voted upon, using a modified Delphi process. Overall, 5310 articles were screened, 171 articles were analysed, and 65 were used to derive recommendations. These articles included six randomized controlled trials plus cohort studies and case series. The highest level of evidence concerned dosing recommendations for topical clindamycin in mild disease (with systemic tetracyclines for more frequent/widespread lesions) and biologic therapy (especially adalimumab) as second‐line agents (following conventional therapy failure). Good‐quality evidence was available for the hidradenitis suppurativa clinical response (HiSCR) as a dichotomous outcome measure in inflammatory areas under treatment. Lower‐level evidence supported recommendations for topical triclosan and oral zinc in mild‐to‐moderate HS, systemic clindamycin and rifampicin in moderate HS and intravenous ertapenem in selected patients with more severe disease. Intralesional or systemic steroids may also be considered. Local surgical excision is suggested for mild‐to‐moderate HS, with wide excision for more extensive disease. Despite a paucity of good‐quality data on management decisions in HS, this systematic review has enabled the development of robust and easily applicable clinical recommendations for international physicians based on graded evidence.
Introduction
Hidradenitis suppurativa (HS)/acne inversa is a debilitating, chronic, inflammatory skin disease that, for the most part, remains poorly understood and managed. The prevalence of HS is estimated to be around 1% of the general population, it occurs more frequently in females than males, and it usually presents after puberty.1 Keratin plugging of hair follicles and recurrent inflammation lead to the formation of painful nodules and abscesses that rupture, causing sinus tracts (tunnels) and scarring.1, 2 Patients often experience long delays before an HS diagnosis is established.2 Moreover, the disease is often associated with a significant negative impact on quality of life.2
Hidradenitis suppurativa is difficult to treat, and clinical practice is variable; therefore, there remains a large unmet medical need in this area. Evidence‐based treatment guidelines for HS are rare; the original S1 guidelines developed by the European Dermatology Forum,1 a revised version including graded levels of evidence and first‐, second‐ and third‐line treatment recommendations,2 and national guidance publications3, 4, 5 are some of the few resources currently available. There is a need for international, evidence‐based consensus on management of HS that is widely and easily applicable in clinical practice.
The aim of the HS ALLIANCE working group was to develop international consensus recommendations for the treatment and management of patients with HS, which go beyond the current guidelines and provide practical suggestions on how tools and treatments should be used. The recommendations cover key areas identified by the International Steering Committee (ISC) as imperative to address, including comorbid diseases, antibiotic and biologic therapies, surgical interventions and monitoring.
Methods
HS ALLIANCE working group
The working group consists of an ISC of experts in the field of dermatology and dermatologic surgery, bibliographic fellows (nominated by the ISC) and national faculty members (known physicians with an interest in HS; Appendix S1). Together, they represent 25 countries worldwide.
The ISC identified a number of key areas for clinical focus in HS and defined a list of clinical questions that should be answered in order to develop consensus recommendations, using a modified Delphi consensus process (Fig. 1). These questions were ranked and refined by the national faculty, clinical care gaps were identified, and a final list of nine overarching questions was selected.
Figure 1.
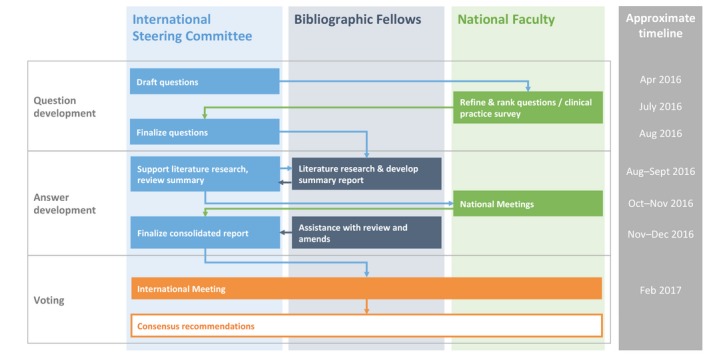
Representation of the modified Delphi process used to reach consensus.
To address these questions, systematic literature searches were performed by the bibliographic fellows, supported by the ISC and a report containing draft evidence‐based statements developed for review by the national faculty. Several hundred comments were reviewed and incorporated by the ISC (assisted by the bibliographic fellows), and a final consolidated report was prepared for the working group. The report was then discussed at a face‐to‐face meeting (Copenhagen, Denmark, on 10 and 11 February 2017), at which the group refined and voted upon the draft recommendations.
Literature search
For each clinical question to be answered (nine in total), a bibliographic fellow performed a systematic literature search. Search terms and strings were constructed and tested in conjunction with the ISC. Search strings incorporated both Medical Subject Headings (MeSH) and free text key words (Appendix S1). Databases searched include PubMed/Medline, The Cochrane Library, EMBASE, CINAHL, SCOPUS, BIOSIS and Web of Science. Further manual searches were conducted, as required, for recent abstracts, trial registries and relevant articles from reference lists.
Included study designs comprised randomized controlled trials (RCTs), prospective and retrospective studies, case series with ≥10 cases and other peer‐reviewed articles. Animal studies, single case studies or case series with <10 patients, narrative reviews, editorials and non‐peer‐reviewed articles were excluded. Studies considered had to be published between August 1996 and the date of this review (August 2016), and be in English language.
Identified articles underwent a two‐stage screening process: firstly, titles and abstracts were scanned for relevance to the clinical question being investigated, and secondly, full‐text copies of relevant articles were obtained and reviewed against the inclusion and exclusion criteria, and any duplicate, low‐quality or outdated articles were excluded.
Included articles had the following data extracted: study characteristics, including design, setting/data source and study period; participant characteristics, such as sample size, mean age, sex, mean follow‐up (if applicable) and location of the disease; baseline (pretreatment) characteristics, where applicable; severity of HS and treatment history to assess homogeneity within populations; treatment, where applicable, including type, dosage and length of treatment; and outcomes after treatment, where applicable (defined per question).
Evidence was rated by the bibliographic fellows working on each clinical question (individuals or pairs), using the Oxford Centre for Evidence‐based Medicine criteria (http://www.cebm.net/index.aspx?o=1025). Data extraction and reporting were performed according to the 2009 PRISMA statement (http://www.prisma-statement.org/PRISMAStatement/PRISMAStatement.aspx) (Appendix S2).
Development of evidence‐based statements
A report summarizing the collected evidence was prepared for each question, including evidence ratings and draft evidence‐based statements; this was reviewed by the ISC before sending out to the national faculty for review. Each participating nation organized a local faculty meeting, at which the faculty indicated their agreement with the draft statements, discussed any concerns and suggested any wording amends. These discussions and revisions were captured in a meeting report from each nation and were passed to the ISC for review. The ISC considered all national faculty feedback and prepared a consolidated report with revised statements for each clinical question. The consolidated report was sent to the working group for review ahead of the international meeting, at which the ISC and national faculty representatives voted on each evidence‐based statement.
Voting process
Individuals assigned each statement an ‘agreement score’ between 1 (lowest) and 9 (highest), and these scores were collated into one of three ranges: 1–3, 4–6 and 7–9. Level of agreement was indicated by the percentage of individuals scoring within the 7–9 range; if ≥75% of individuals scored a statement within the 7–9 range, agreement was recorded. If no agreement was achieved, the statements were discussed and amended with agreement from the ISC co‐chairs, and voting took place again. If agreement was not achieved after a second vote, a lack of agreement was recorded. One vote was allowed per country among the national faculty members, and one vote for each member of the ISC.
Results
In total, 5310 articles were screened and 171 were included in the data analysis. Very few RCTs have been conducted in HS; therefore, many of the available data were from case series or expert opinion (grade 4 or 5 evidence).
Assessment of comorbidities
Fewer than 20 articles were available to guide practice around assessment of comorbidities in patients with HS; nonetheless, much of the available evidence was high grade (level 1 or 2). Recommendations on assessment of comorbid disorders in patients with HS are presented in Table 1 and summarized below.
Table 1.
Recommendations for assessment of comorbid disorders in patients with HS
| What comorbidity‐related screening should be assessed in patients with HS? | |
|---|---|
Comorbidities and risk factors for HS include (evidence level, grade of recommendation):
|
Consensus (93%)
|
| Weight loss/reduction in body mass index (obese patient; BMI ≥ 30) can be effective in reducing severity of disease in the long term12, 16 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| When assessing patients, particular emphasis should be paid to psychological comorbidity18 (evidence level 5, grade of recommendation D). | Consensus (100%)
|
| In patients with chronic perianal and perineal HS, and in particular in the presence of fistulas, the possibility of Crohn's disease should be considered21 (evidence level 5, grade of recommendation D). | Consensus (96%)
|
| The potential for malignant transformation in patients with chronic HS should be recognized20 (evidence level 5, grade of recommendation D). | Consensus (100%)
|
The association between HS and smoking is particularly strong, although a causal relationship has not been ascertained. An increased risk of metabolic syndrome in HS is recognized,6, 7, 8, 9 with associated increases in risk for cardiovascular (CV) events and mortality; notably, the risk of CV death is 58% higher in patients with HS than in patients with severe psoriasis.10 Obesity is also a well‐established independent risk factor for HS,11, 12, 13, 14, 15 with improvements in disease severity demonstrated after weight loss.12, 16, 17 Reflecting the psychological and physical burden of the disease, rates of depression and anxiety among HS patients are significantly increased compared with age‐ and gender‐matched controls.18
Other severe inflammatory conditions, such as Crohn's disease and spondyloarthropathy, can co‐exist in HS patients.19 In addition, HS lesions can undergo malignant transformation into squamous cell carcinoma. This is a rare occurrence, but is associated with a high mortality rate.20
Nonetheless, an association with comorbidity does not infer a recommendation for widespread screening in HS; clinicians should adhere to the latest population‐based screening recommendations in distinct populations, e.g. screening for metabolic syndrome in obese patients.
Treatment
Medical therapy
Apart from topical clindamycin and systemic tetracycline, the evidence for efficacy of non‐biologic agents in HS comes from level 4 studies and expert opinion, including the previous European S1 guidelines. The size and design of studies, as well as the absence of long‐term data for non‐biologic treatments, make standardized recommendations on optimal management challenging (Table 2).
Table 2.
Recommendations for medical treatment of HS
| Which non‐biologic therapies are effective in the short, medium and long term for the treatment of HS? | |
| There are very few long‐term data26, 33, 34 | Consensus (100%)
|
| In Hurley stage II/III patients presenting with several active lesions, systemic clindamycin and rifampicin (dosage: 300 mg twice daily) should be administered for an average length of 10 weeks24, 25, 26, 27 (evidence level 4, grade of recommendation C).Local antibiotic guidelines should be followed The S1 European guidelines recommend that antibiotics should be used for up to 3 months and reintroduced in case of recurrence under the requirement that they were effective at the last time of use1 (evidence level 5, grade of recommendation D) | Consensus (100%) [after revote]
|
| Systemic acitretin may be considered as a third‐line therapy for patients with mild/moderate HS35, 36, 37, 38 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| Which antibiotics are and are not efficacious for the treatment of HS and how should they be used? | |
| In Hurley stage I/II patients with mild localized HS with few lesions, topical clindamycin 1% is a possible therapy, especially in the absence of deep inflammatory lesions (abscesses).23 The topical formulation may be administered twice daily for a maximum of 3 months. Resistance to clindamycin has changed since reviewed studies were completed; therefore, local antibiotic guidelines should be followed (evidence level 2, grade of recommendation B). | Consensus (92%) [after revote]
|
| In Hurley stage I/II patients presenting with several lesions and frequent exacerbations, the therapeutic group of systemic tetracyclines may be considered23 (evidence level 2, grade of recommendation B).However, many countries use other derivatives from the same group (e.g. doxycycline, minocycline), for which there is no high‐level evidence. Only one antibiotic of the same class should be used for a maximum of 12 weeks. Local antibiotic guidelines should be followed. | Consensus (100%)
|
| In Hurley stage II/III patients presenting with several active lesions, systemic clindamycin and rifampicin (dosage: 300 mg twice daily) should be administered for an average length of 10 weeks24, 25, 26, 27 (evidence level 4, grade of recommendation C).Local antibiotic guidelines should be followed. | Consensus (96%)
|
| A triple regimen of rifampicin (10 mg/kg once daily), moxifloxacin (400 mg once daily) and metronidazole (500 mg thrice daily) administered for up to 12 weeks, with metronidazole discontinuation at week 6, may offer efficacy in Hurley stage I and II patients, but should be used with appropriate monitoring39 (evidence level 4, grade of recommendation C).Local antibiotic guidelines should be followed. | Consensus (81%)
|
| In selected patients with severe HS, a 6‐week course of intravenous ertapenem (1 g daily) with consolidation treatment of rifampicin/moxifloxacin/metronidazole may be considered40 (evidence level 4, grade of recommendation C).Local antibiotic guidelines should be followed. | Consensus (88%)
|
Antibiotics studied in HS (evidence level, grade of recommendation):
|
Consensus (96%)
|
| There is no evidence for the use of other antibiotics | Consensus (100%)
|
| The S1 European guidelines recommend that antibiotics should be used for up to 3 months and reintroduced in case of recurrence under the requirement that they were effective at the last time of use1 (evidence level 5, grade of recommendation D). | Consensus (100%)
|
| In HS, microbiological cultures are not useful (evidence level 5, grade of recommendation D) | Consensus (92%)
|
| How and when in the disease course of HS should biologics be introduced? | |
| There are few RCTs and little high‐quality evidence | Consensus (100%)
|
| Adalimumab should be considered as first‐choice biologic agent in moderate/severe HS after failure of conventional treatments28, 29, 30 (evidence level 2, grade of recommendation B). | Consensus (100%)
|
| Infliximab has also been shown to be effective and should be considered as a second‐line biologic for moderate/severe HS31 (evidence level 2, grade of recommendation B). | Consensus (81%)
|
| Anakinra (evidence level 2, grade of recommendation B) has also been shown to be effective and should be considered as a third‐line biologic for moderate/severe HS32 Ustekinumab (evidence level 4, grade of recommendation C) is a potentially effective treatment for moderate/severe HS42 | Consensus (84%)
|
| Etanercept is not effective for the treatment of HS43 (evidence level 2, grade of recommendation B). | Consensus (96%)
|
| When should (any) combinations of medical treatments be considered for patients with HS and what combinations should be used? | |
| Evidence for the use of combination therapy in HS is limited | Consensus (92%)
|
| In Hurley stage II/III patients presenting with several active lesions, systemic clindamycin and rifampicin (dosage: 300 mg twice daily) should be administered for an average length of 10 weeks24, 25, 26, 27 (evidence level 4, grade of recommendation C).Local antibiotic prescribing guidelines should be followed. | Consensus (100%) [after revote]
|
| A triple regimen of rifampicin (10 mg/kg once daily), moxifloxacin (400 mg once daily) and metronidazole (500 mg thrice daily) administered for up to 12 weeks, with metronidazole discontinuation at week 6, may offer efficacy in Hurley stage I and II patients, but should be used with appropriate monitoring39 (evidence level 4, grade of recommendation C).Local antibiotic prescribing guidelines should be followed. | Consensus (100%) [after revote]
|
| In Hurley stage I/II, the combination of oral zinc gluconate (30 mg thrice daily) and topical triclosan 2% (twice daily) may be considered as a treatment option44 (evidence level 4, grade of recommendation C). | Consensus (81%)
|
| Intralesional steroids may be helpful for acute inflammatory nodules in combination with other treatments at all Hurley stages45 (evidence level 4, grade of recommendation C). | Consensus (80.8%)
|
| Low‐dose systemic corticosteroids (10 mg prednisolone equivalent per day) may be an effective adjunct in recalcitrant HS46 (evidence level 4, grade of recommendation C).Long‐term corticosteroid treatment should be used with appropriate caution. | Consensus (96%)
|
The evidence‐based literature available on the use of antibiotics in HS is limited and largely restricted to retrospective studies. The combination of systemic clindamycin and rifampicin is by far the most investigated treatment. For Hurley stage I/II, topical clindamycin 1% is a possible therapy, especially in the absence of abscesses.22, 23 If there are several lesions and frequent exacerbations, the therapeutic group of systemic tetracyclines may be considered.23 A triple regimen of rifampicin (10 mg/kg once daily), moxifloxacin (400 mg once daily) and metronidazole (500 mg thrice daily) administered for up to 12 weeks, with metronidazole discontinuation after 6 weeks, may be an alternative option. In Hurley stage II/III patients who have several active lesions, systemic clindamycin and rifampicin (dosage: 300 mg twice daily) should be administered.24, 25, 26, 27
Six RCTs assessing the use of biologic therapy have been performed to date in HS (four of them with adalimumab), and two more are ongoing. Recommendations for the use of these agents are based on the results of these trials. Adalimumab should be considered as the first‐choice biologic agent in moderate/severe HS after failure of conventional treatments,28, 29, 30 followed by infliximab31 and anakinra32 as second‐ and third‐line options, respectively.
Evidence for combination therapies is very limited and largely focused on small case series or retrospective reviews of antibiotics, topical triclosan and oral zinc or steroid‐based therapies.
Surgical therapy
The evidence for surgical therapies in HS is based on case series and cohort studies with differing methodologies and outcome definitions (low‐grade evidence; Table 3).
Table 3.
Recommendations for surgical treatment of HS
| Which types of surgery may benefit patients with HS? | |
| Case and cohort studies used variable definitions of recurrence and a wide range of follow‐up time and, therefore, cannot be compared. | Consensus (100%)
|
| In acute situations, surgical incision and drainage of tense and painful abscesses, i.e. fluctuant lesions, may be performed. However, incision and drainage should not be considered as a sole treatment because recurrence is almost inevitable47, 48, 49, 50 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| Surgical procedures, such as limited excision, deroofing and STEEP, can be used for solitary lesions of the disease. They could be performed for recurrent HS lesions at fixed locations or fistula/sinus tract formation in limited areas51 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| Wide excision of the entire affected area, with removal of (non‐)inflamed sinuses, nodules and scar tissue, may be performed in Hurley stage III to prevent recurrence53, 54, 55 (evidence level 4, grade of recommendation C). | Consensus (96%)
|
| Chronic HS lesions that have not shown any signs of inflammation for a prolonged period of time may be excised to prevent further recurrence56, 57 (evidence level 5, grade of recommendation D). | Consensus (78%)
|
| Special attention should be paid to patients with perianal and/or perineal HS due to the possible existence of anal, urethral and vaginal fistulas and presence of squamous cell carcinoma47, 58 (evidence level 4, grade of recommendation C). | Consensus (92.6%)
|
| CO2 ablative laser treatment is an effective alternative method to electrosurgical or cold steel techniques59, 60 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| How should medical and surgical treatments be integrated? | |
| There are no RCTs describing the combination of medical and surgical treatments | Consensus (92.6%)
|
| Pre‐ and postoperative biologic therapy may lead to a lower recurrence rate and a longer disease‐free interval61, 62 (evidence level 4, grade of recommendation C). | Consensus (89%)
|
| There is no current literature regarding adverse events when integrating biologic therapy and surgery in HS patients. Studies in other immune‐mediated diseases are insufficient to advise preoperative interruption of biologics (evidence level 5, grade of recommendation D). | Consensus (100%)
|
| Adalimumab reduces the need for surgical procedures (incisions and drainage)29 (evidence level 2, grade of recommendation C). | Consensus (85.7%)
|
The type of surgery performed and the required margins are based on the severity of the disease.1 For limited disease with solitary lesions, individual abscesses may be drained as a supplemental measure,47, 48, 49, 50 and surgical procedures, such as limited excision, deroofing and STEEP, can be performed.51 For tense and painful abscesses, no medical therapy will be effective and surgical drainage may be required;52 however, this should not be considered as sole treatment, because recurrence is almost inevitable. Widespread, severe disease requires more extensive excision.53, 54, 55 Chronic inactive lesions can be removed to prevent recurrence.56, 57
There is limited evidence for the combination of medical and surgical therapies in HS. Studies in other immune‐mediated diseases (psoriasis, psoriatic arthritis, rheumatoid arthritis and inflammatory bowel disease) are insufficient to advise on preoperative interruption of biologics (Appendix S1).
Monitoring disease activity and response to therapy
Within the 13 RCTs performed in patients with HS, 90% of the outcome measures lacked validated data to support their clinical applicability. Seven studies of six different HS outcome measures have provided validation data;11, 14, 63, 64, 65, 66, 67 however, one measure [the acne inversa severity index (AISI)]64 is yet to be employed in an RCT. Hurley stage measurements and Sartorius scoring are widely used assessments of severity; however, these have limitations and are only partially validated.14, 63, 67 Validated outcome measures that may be used to assess changes in disease over time or following treatment include the hidradenitis suppurativa clinical response (HiSCR),66 which is supported by good‐quality evidence, and more general measures encompassing physician‐ and patient‐reported levels of extent, pain and quality of life.11, 64, 65
High‐level trial data for use of adalimumab in patients with HS suggest that those patients who do not respond to treatment (<25% improvement in abscesses and inflammatory nodules) within 12 weeks should discontinue the drug, while partial or good responders should continue the therapy with ongoing assessment.28, 29, 68, 69 Evidence is lacking for other biologic therapies, and any clinical decisions should be based on close monitoring and risk : benefit assessments.
The working group recommendations for assessment of disease and monitoring are given in Table 4.
Table 4.
Recommendations for assessment of disease and monitoring
| How should disease activity and response to treatment be monitored in patients with HS? | |
| The majority of the outcome measurement instruments used in HS RCTs lack substantial validation evidence. Furthermore, a validated measure for baseline severity assessment is also unexplored. This may hamper comparisons of HS trials investigating future treatment regimens | Consensus (100%)
|
| Hurley staging is suggested by experts for assessment of baseline severity, especially with regard to the extent of scarring. It is, however, not a dynamic tool and so it should only be used to describe an area affected by HS (and not to define overall severity of disease). Each individual area affected by HS should be assessed independently (evidence level 5, grade of recommendation D). | Consensus (96%)
|
| The HiSCR is supported by good‐quality validation studies and is recommended to be used as a dichotomous outcome measure in inflammatory areas under treatment66 (evidence level 2, grade of recommendation B). | Consensus (100%)
|
| Patient‐reported outcome measures (e.g. DLQI, VAS) should be included in the overall assessment of the HS patient as they may offer important insight on functioning, quality of life and symptoms (e.g. pain and itching)63, 64 (evidence level 4, grade of recommendation C). | Consensus (100%)
|
| The modified Sartorius score has been partially validated and can be used to assess severity14, 67 (evidence level 4, grade of recommendation C). | Consensus (96%)
|
| How long should biologics be used in patients responding/not responding? | |
Adalimumab28, 29, 68, 69:
|
Consensus (92.6%)
|
| Not enough published data are available for other biologics. Decisions about whether and how to continue treatment should be based on a close monitoring of patients and careful assessment of the risk : benefit ratio (evidence level 5, grade of recommendation D). | Consensus (96%)
|
DLQI, dermatology life quality index; VAS, visual analogue scale.
Discussion
This systematic literature review demonstrates the paucity of an evidence base for clinical decision‐making in HS. This is both in terms of the number of studies and the quality of the data (Appendix S3). The highest level evidence available addresses the use of topical and systemic antibiotics and biologic therapies, especially adalimumab. The majority of the remaining evidence to guide management decisions is based on case reports, small cohort studies and expert opinion. The dearth of high‐quality evidence in HS strengthens the need for practical expert consensus recommendations to optimize management worldwide and improve care for these patients. The HS ALLIANCE of international experts has reviewed the available data and has developed these consensus clinical recommendations that are both pragmatic and evidence‐based.
Only 13 RCTs have been performed in HS; of these, seven have not formed the basis of treatment recommendations made in this review. This is due to the inclusion criteria of the review, and limited clinical applicability or patient numbers (see Appendix S1 for details).
Intense pulsed light and photodynamic therapy studies provide limited data for the treatment of HS. Their applicability to widespread practice and outcomes is currently unknown. Further research is warranted for these promising supplemental therapies.
Topical clindamycin may be used in early HS; however, real‐world resistance to clindamycin tends to be higher than suggested in a RCT, and the efficacy of topical clindamycin Hurley stage II is questioned by many physicians. Systemic tetracyclines are an alternative option, but these are no longer available in some countries. The combination of 100 mg minocycline administered orally once per day and 0.5 mg colchicine administered twice per day for 6 months, followed by maintenance 0.5 mg colchicine for 3 months, has recently been shown to provide substantial improvement or remission in a prospective pilot study in 20 patients;70 further investigation of this combination is warranted. For Hurley stage II/III HS, the use of systemic antibiotic combinations is common, despite there being only low‐grade (level 4) evidence to support this. Furthermore, the US Food and Drug Administration has recommended that fluoroquinolone antibiotics be reserved for patients with acute infections who have no alternative treatment options, due to a poor risk : benefit profile (https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm). Long‐term use of any antibiotic is not recommended.
The use of intravenous ertapenem is included in this review as a treatment option in selected severe patients, but its use is at the discretion of the treating physician; the study on which this recommendation is based included patients who were not surgical candidates, or who had been refused a surgical intervention.40 This study was given the same grading as the evidence for systemic clindamycin and rifampicin;24, 25, 26, 27 however, the working group considered the recommendation for ertapenem to be less strong.
Adalimumab is considered the first‐line biologic therapy in HS due to the amount of high‐level evidence available to support its use. The indicated dose of adalimumab in HS is 160 mg on day 1, 80 mg on day 15 and a single 40 mg injection every week from week 4 onwards. This is a higher dosing schedule than that for plaque psoriasis, in which the recommended regimen is 80 mg on day 1, 40 mg on day 8 and 40 mg every other week thereafter. Infliximab also has high‐level evidence to support its use; nonetheless, the primary outcome of the RCT investigating infliximab was neither validated nor achieved. The dosing schedule for infliximab is 5 mg/kg body weight on day 0, 2 and 6 and then every 8 weeks. Anakinra was considered by the group as third‐line, despite having the same grade of evidence as infliximab; this was due to the study including fewer patients and different outcomes. Also, earlier studies of anakinra had been less convincing than those for infliximab. Case reports of etanercept suggest efficacy in HS; however, the performed RCT showed no effect, and our recommendation is based on this high‐level evidence. Secukinumab was not included in these recommendations due to lack of evidence at the time of the literature search; however, a pilot study for this drug in HS is now underway (ClinicalTrials.gov Identifier: NCT03099980).
Many physicians managing HS routinely collect blood and skin samples to assess bacterial cultures and treat their patients according to the results. Traditional microbiological sampling is not useful in HS and should not influence treatment decisions, as there is no evidence for infection as a causal factor. Antibiotics are prescribed in HS for their anti‐inflammatory properties, rather than for their antibacterial action; this may be an essential point for physicians in their understanding of the pathogenesis of HS. Likewise, antiseptics are commonly prescribed with no evidence base to support their use, except for reducing smell.
The modified Sartorius score (MSS) has been partially validated as a tool to assess disease severity; however, it can be considered time‐consuming to use, and several versions of this score have been developed with inconsistent use of terminology, leading to confusion among physicians. For example, modified HS score (modified HSS), MSS and HS‐LASI (HS lesion, area and severity index) are all used.14, 29, 71, 72 The HiSCR has more validation data than other traditional severity tools; however, a further validated tool to assess cross‐sectional severity in HS has recently been developed.73
The HS ALLIANCE working group highlighted a number of future research topics for HS during consensus discussions. Foremost, the group felt that new RCTs investigating individual or combination therapies for HS were needed and that these should include appropriate and validated outcome measures, patient‐reported outcomes and long‐term data. The effect of treatments for HS on associated CV risk factors was also an area of potential interest, especially considering that use of metformin and anti‐TNF‐alpha agents in other diseases has been associated with reduced CV risk.74, 75 Evidence to guide optimal practice for integrating medical and surgical therapies is also required, along with further validation of outcome measures to assess changes in HS activity over time and with treatment response.
The paucity of high‐level evidence on which to base clinical recommendations, especially regarding optimal treatment strategies for long‐term and CV outcomes, was a limitation to this systematic review. Furthermore, multiple literature reviewers were involved, meaning that despite the same objectives and criteria, slight differences occurred in the way the search strings were designed and the data reviews were executed. Due to the design of the consensus process, the literature searches were performed in advance of the recommendations being finalized and this review being written; therefore, some of the latest evidence, including preliminary studies of spironolactone, ustekinumab and apremilast, was not considered within the recommendations. We would recommend that an update to these recommendations and evidence grades is performed once ongoing RCTs have reported, including any additional data that were not captured here.
The HS ALLIANCE recommendations in this review largely agree with the current clinical practice guidelines in Europe,1, 2 suggesting similar approaches for first‐ and second‐line medical and surgical treatment options (Appendix S1; comparison of current recommendations). The German guidelines and the recent Swiss and Canadian guidance3, 4, 5 incorporated existing evidence and practitioners’ expertise into a framework for the evaluation and treatment of patients with HS, in a non‐systematic fashion. Nonetheless, the discussed medical and surgical therapies align with those in the European S1 guidance, albeit with a greater focus on hormonal and laser therapies in Canada. The original European S1 guideline1 and the German and Swiss guideline offered no methodology for creation of the recommendations, but declared funding and conflict of interests from the guideline groups. Building on these, Gulliver et al.2 reviewed and graded the available evidence behind the therapeutic recommendations and created an algorithm for management of HS, while providing more transparent methodology and less conflict information. The current guidelines employed both a systematic, consensus approach and a high level of transparency to generate recommendations that address previously unanswered questions in clinical practice and are clear to implement. A summary of the guideline ratings is available in the Appendix S1.
In conclusion, there is currently a paucity of research evidence available for HS, in terms of numbers of studies and quality of data. Nonetheless, this systematic literature review captures and grades the available evidence in order to provide applicable recommendations on important clinical questions for healthcare professionals managing HS. The current international guidance goes beyond the regional recommendations available, providing a comprehensive and transparent set of consensus guidelines for HS.
Author contributions
CCZ, FB, WG, BH, ABK, BK, AM, MP, EPP, TT, HHvdZ and GBEJ are all members of the ISC, have developed key questions to be answered, collaborated with the bibliographic fellows for literature searches, revised and voted on consensus statements, and substantially reviewed and approved drafts of this manuscript. CCZ and GBEJ were co‐chairs of the ISC. RH, HCR, KRvS, JLD‐B and ARJVV are bibliographic fellows – they conducted the literature searches, with input from ISC members, revised the consensus statements and have substantially reviewed and approved drafts of this manuscript. All authors had full access to data and materials in order to develop this manuscript, made substantial contributions to the article or critically revised it for important intellectual content, and approved the final version for submission.
Collaborators
Members of the wider HS ALLIANCE faculty from each nation involved are listed in the Appendix S1. The national faculty involved in reviewing and revising recommendations, and a representative from each nation participated in consensus voting.
Other contributions
Medical writing support in the development and revision of this manuscript (preparation of manuscript drafts for the named authors to edit) was provided by Claire Price of Lucid UK, and this support was funded by AbbVie Inc.
Supporting information
Appendix S1. Members of the HS ALLIANCE national faculties.
Appendix S2. Search strings and PRISMA diagrams for each clinical question literature search.
Appendix S3. Comparison of current recommendations with existing clinical practice guidelines and JAMA rating of guidelines.
Conflicts of interest
The authors have made the following disclosures: CCZ received honoraria from AbbVie, Bayer Healthcare, Biogen and PPM for participation as an advisor and speaker; from Allergan, Almirall, Celgene, GSK, Inflarx, Novartis and UCB for participation as an advisor; and from Jenapharm and Pierre Fabre for participation as a speaker. His department has received grants from AOTI, Astra Zeneca, Biogen, Celgene, Dr. Reddy's Laboratories, Galderma, Novartis and UCB for his participation as an investigator. FGB received honoraria from AbbVie for participation as an advisor and speaker. His department has received grants from AbbVie and Novartis for his participation as an investigator. WG – relationships with commercial interests (since 2015): received grants/research support from AbbVie, Amgen, Eli Lilly, Novartis and Pfizer; honoraria for advisory boards/invited talks/consultation from AbbVie, Actelion, Amgen, Arylide, Boehringer, Celgene, Cipher, Eli Lilly, Galderma, Janssen, Novartis, Pfizer and Tribute; and clinical trials (study fees) from AbbVie, Astellas, Celgene, Galderma, Janssen, LEO Pharma, Novartis, Pfizer and Regeneron. BH received fees from AbbVie, Novartis, UCB Pharma, Solenne BV and Janssen‐Cilag for consultation/advisory, scientific research, congress and courses; and fees from Novartis for consultation/advisory, scientific research and congress. ABK has acted as consultant and investigator for AbbVie and UCB; and consultant for Novartis and Celtaxsys. BK received research support from or has acted as a principal investigator for AbbVie, Pfizer, Merck Sharp & Dohme and Novartis; has acted as consultant for Merck Sharp &Dohme, Pfizer, Janssen, AbbVie, Novartis, Celgene, Leo and Lilly; received honoraria from Janssen, Pfizer, AbbVie, Novartis, Celgene, Lilly and Leo; and has acted as scientific advisory board member for Pfizer, AbbVie, Almirall, Novartis, Celgene, Janssen, Lilly and Leo. AM received honoraria from AbbVie, Celgene, Janssen, Novartis, Merck Sharp & Dohme, ISDIN, UCB and Pfizer for participation on advisory boards, as well as acting as a consultant and speaker. MP received honoraria, educational grants or consultancies from AbbVie, Allergan, Beiersdorf, BMS, Galderma, Janssen‐Cilag, Leo Pharma, L'Oreal, Novartis, MSD and UCB. EP has acted as consultant, speaker, principal investigator or received grants from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Janssen‐Cilag, Novartis, Pfizer and UCB. HCR received support from AbbVie for participation as a bibliographic fellow in the HS ALLIANCE programme. TT has acted as advisory board member for AbbVie. HHvdZ has acted as advisory board member for AbbVie and InflaRX. GBEJ has acted as advisory board member, principal investigator or received research grant from AbbVie; has acted as consultant for Astion Pharma; as consultant for Inflarx; has acted as advisory board member for Janssen; has acted as advisory board member, as principal investigator and received research grant from Leo pharma; has acted as advisory board member and consultant, and received research grant from Novartis; has acted as principal investigator for Serono; and has acted as consultant and principal investigator for UCB. ARJVV, JLD‐B, KRvS and RH declared no conflict of interests.
Funding source
The HS ALLIANCE programme is led by the ISC and is supported and funded by AbbVie. AbbVie selected experts for participation in the HS ALLIANCE, provided funding to invited participants, including honoraria for their attendance at the meetings (to allow discussion and alignment as part of the consensus process) and reimbursed travel to and from the consensus meetings. No payments were made to the authors for the development of this manuscript. The authors maintained complete control over the manuscript content, and it reflects their opinions. AbbVie reviewed the final manuscript draft for scientific accuracy, but was not involved in methodology, data collection and analysis, or drafting.
References
- 1. Zouboulis CC, Desai N, Emtestam L et al European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29: 619–644. [DOI] [PubMed] [Google Scholar]
- 2. Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence‐based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016; 17: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alavi A, Lynde C, Alhusayen R et al Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg 2017; 21: 513–524. [DOI] [PubMed] [Google Scholar]
- 4. Zouboulis CC, Bechara FG, Fritz K et al S1‐Leitlinie zur therapie der hidradenitis suppurativa/acne inversa* (ICD‐10 Ziffer: L73.2). J Dtsch Dermatol Ges 2012; 10: s1–s31. [DOI] [PubMed] [Google Scholar]
- 5. Hunger RE, Laffitte E, Läuchli S et al Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology 2017; 233: 113–119. [DOI] [PubMed] [Google Scholar]
- 6. Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol 2014; 70: 699–703. [DOI] [PubMed] [Google Scholar]
- 7. Riis PT, Soeby K, Saunte DM, Jemec GB. Patients with hidradenitis suppurativa carry a higher systemic inflammatory load than other dermatological patients. Arch Dermatol Res 2015; 307: 885–889. [DOI] [PubMed] [Google Scholar]
- 8. Sabat R, Chanwangpong A, Schneider‐Burrus S et al Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS One 2012; 7: e31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shalom G, Freud T, Harman‐Boehm I, Polishchuk I, Cohen AD. Hidradenitis suppurativa and metabolic syndrome: a comparative cross‐sectional study of 3207 patients. Br J Dermatol 2015; 173: 464–470. [DOI] [PubMed] [Google Scholar]
- 10. Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all‐cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol 2016; 152: 429–434. [DOI] [PubMed] [Google Scholar]
- 11. Canoui‐Poitrine F, Revuz JE, Wolkenstein P et al Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol 2009; 61: 51–57. [DOI] [PubMed] [Google Scholar]
- 12. Kromann CB, Ibler KS, Kristiansen VB, Jemec GB. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol 2014; 94: 553–557. [DOI] [PubMed] [Google Scholar]
- 13. Revuz JE, Canoui‐Poitrine F, Wolkenstein P et al Prevalence and factors associated with hidradenitis suppurativa: results from two case‐control studies. J Am Acad Dermatol 2008; 59: 596–601. [DOI] [PubMed] [Google Scholar]
- 14. Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009; 161: 831–839. [DOI] [PubMed] [Google Scholar]
- 15. Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population‐based study of Olmsted County, Minnesota. J Invest Dermatol 2013; 133: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol 2014; 39: 315–317; quiz 317–318. [DOI] [PubMed] [Google Scholar]
- 17. Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol 2016; 30: 895–896. [DOI] [PubMed] [Google Scholar]
- 18. Shavit E, Dreiher J, Freud T, Halevy S, Vinker S, Cohen AD. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2015; 29: 371–376. [DOI] [PubMed] [Google Scholar]
- 19. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol 2010; 2: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pena ZG, Sivamani RK, Konia TH, Eisen DB. Squamous cell carcinoma in the setting of chronic hidradenitis suppurativa; report of a patient and update of the literature. Dermatol Online J 2015; 21(4): Retrieved from https://escholarship.org/uc/item/9q9707dp. [PubMed] [Google Scholar]
- 21. Kamal N, Cohen BL, Buche S, Delaporte E, Colombel JF. Features of patients with Crohn's disease and hidradenitis suppurativa. Clin Gastroenterol Hepatol 2016; 14: 71–79. [DOI] [PubMed] [Google Scholar]
- 22. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983; 22: 325–328. [DOI] [PubMed] [Google Scholar]
- 23. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998; 39: 971–974. [DOI] [PubMed] [Google Scholar]
- 24. Bettoli V, Zauli S, Borghi A et al Oral clindamycin and rifampicin in the treatment of hidradenitis suppurativa‐acne inversa: a prospective study on 23 patients. J Eur Acad Dermatol Venereol 2014; 28: 125–126. [DOI] [PubMed] [Google Scholar]
- 25. Gener G, Canoui‐Poitrine F, Revuz JE et al Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology 2009; 219: 148–154. [DOI] [PubMed] [Google Scholar]
- 26. Mendonca CO, Griffiths CE. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol 2006; 154: 977–978. [DOI] [PubMed] [Google Scholar]
- 27. van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology 2009; 219: 143–147. [DOI] [PubMed] [Google Scholar]
- 28. Kimball AB, Kerdel F, Adams D et al Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157: 846–855. [DOI] [PubMed] [Google Scholar]
- 29. Kimball AB, Okun MM, Williams DA et al Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375: 422–434. [DOI] [PubMed] [Google Scholar]
- 30. Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GB. A double‐blind placebo‐controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol 2011; 165: 391–398. [DOI] [PubMed] [Google Scholar]
- 31. Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double‐blind, placebo‐controlled crossover trial. J Am Acad Dermatol 2010; 62: 205–217. [DOI] [PubMed] [Google Scholar]
- 32. Tzanetakou V, Kanni T, Giatrakou S et al Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol 2016; 152: 52–59. [DOI] [PubMed] [Google Scholar]
- 33. Brocard A, Knol AC, Khammari A, Dreno B. Hidradenitis suppurativa and zinc: a new therapeutic approach. A pilot study. Dermatology 2007; 214: 325–327. [DOI] [PubMed] [Google Scholar]
- 34. Anderson MD, Zauli S, Bettoli V, Boer J, Jemec GB. Cyclosporine treatment of severe hidradenitis suppurativa–a case series. J Dermatolog Treat 2016; 27: 247–250. [DOI] [PubMed] [Google Scholar]
- 35. Boer J, Nazary M. Long‐term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer? Br J Dermatol 2011; 164: 170–175. [DOI] [PubMed] [Google Scholar]
- 36. Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol 2014; 171: 170–174. [DOI] [PubMed] [Google Scholar]
- 37. Puri N, Talwar A. A study on the management of hidradenitis suppurativa with retinoids and surgical excision. Indian J Dermatol 2011; 56: 650–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan MG, Shear NH, Walsh S, Alhusayen R. Acitretin: monotherapy or combined therapy for hidradenitis suppurativa? J Cutan Med Surg 2017; 21: 48–53. [DOI] [PubMed] [Google Scholar]
- 39. Join‐Lambert O, Coignard H, Jais JP et al Efficacy of rifampin‐moxifloxacin‐metronidazole combination therapy in hidradenitis suppurativa. Dermatology 2011; 222: 49–58. [DOI] [PubMed] [Google Scholar]
- 40. Join‐Lambert O, Coignard‐Biehler H, Jais JP et al Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother 2016; 71: 513–520. [DOI] [PubMed] [Google Scholar]
- 41. Yazdanyar S, Boer J, Ingvarsson G, Szepietowski JC, Jemec GB. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology 2011; 222: 342–346. [DOI] [PubMed] [Google Scholar]
- 42. Blok JL, Li K, Brodmerkel C, Horvatovich P, Jonkman MF, Horvath B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol 2016; 174: 839–846. [DOI] [PubMed] [Google Scholar]
- 43. Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol 2010; 146: 501–504. [DOI] [PubMed] [Google Scholar]
- 44. Hessam S, Sand M, Meier NM, Gambichler T, Scholl L, Bechara FG. Combination of oral zinc gluconate and topical triclosan: an anti‐inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci 2016; 84: 197–202. [DOI] [PubMed] [Google Scholar]
- 45. Riis PT, Boer J, Prens EP et al Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol 2016; 75: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 46. Wong D, Walsh S, Alhusayen R. Low‐dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol 2016; 75: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 47. Balik E, Eren T, Bulut T, Buyukuncu Y, Bugra D, Yamaner S. Surgical approach to extensive hidradenitis suppurativa in the perineal/perianal and gluteal regions. World J Surg 2009; 33: 481–487. [DOI] [PubMed] [Google Scholar]
- 48. Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol 2015; 73: S62–S65. [DOI] [PubMed] [Google Scholar]
- 49. Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg 2012; 38: 517–536. [DOI] [PubMed] [Google Scholar]
- 50. Kohorst JJ, Baum CL, Otley CC et al Surgical management of hidradenitis suppurativa: outcomes of 590 consecutive patients. Dermatol Surg 2016; 42: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 51. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue‐saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol 2010; 63: 475–480. [DOI] [PubMed] [Google Scholar]
- 52. Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2009; 23: 985–998. [DOI] [PubMed] [Google Scholar]
- 53. Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mitchell KM, Beck DE. Hidradenitis suppurativa. Surg Clin North Am 2002; 82: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 55. Rompel R, Petres J. Long‐term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg 2000; 26: 638–643. [DOI] [PubMed] [Google Scholar]
- 56. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol 1996; 34: 994–999. [DOI] [PubMed] [Google Scholar]
- 57. Slade DE, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg 2003; 56: 451–461. [DOI] [PubMed] [Google Scholar]
- 58. Bocchini SF, Habr‐Gama A, Kiss DR, Imperiale AR, Araujo SE. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum 2003; 46: 944–949. [DOI] [PubMed] [Google Scholar]
- 59. Lapins J, Sartorius K, Emtestam L. Scanner‐assisted carbon dioxide laser surgery: a retrospective follow‐up study of patients with hidradenitis suppurativa. J Am Acad Dermatol 2002; 47: 280–285. [DOI] [PubMed] [Google Scholar]
- 60. Mikkelsen PR, Dufour DN, Zarchi K, Jemec GB. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: a retrospective study. Dermatol Surg 2015; 41: 255–260. [DOI] [PubMed] [Google Scholar]
- 61. DeFazio MV, Economides JM, King KS et al Outcomes after combined radical resection and targeted biologic therapy for the management of recalcitrant hidradenitis suppurativa. Ann Plast Surg 2016; 77: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van Rappard DC, Mekkes JR. Treatment of severe hidradenitis suppurativa with infliximab in combination with surgical interventions. Br J Dermatol 2012; 167: 206–208. [DOI] [PubMed] [Google Scholar]
- 63. Alavi A, Anooshirvani N, Kim WB, Coutts P, Sibbald RG. Quality‐of‐life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol 2015; 16: 61–65. [DOI] [PubMed] [Google Scholar]
- 64. Chiricozzi A, Faleri S, Franceschini C, Caro RD, Chimenti S, Bianchi L. AISI: a new disease severity assessment tool for hidradenitis suppurativa. Wounds 2015; 27: 258–264. [PubMed] [Google Scholar]
- 65. Highton L, Chan WY, Khwaja N, Laitung JK. Treatment of hidradenitis suppurativa with intense pulsed light: a prospective study. Plast Reconstr Surg 2011; 128: 459–465. [DOI] [PubMed] [Google Scholar]
- 66. Kimball AB, Jemec GB, Yang M et al Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014; 171: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 67. Sartorius K, Killasli H, Heilborn J, Jemec GB, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol 2010; 162: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 68. Sotiriou E, Goussi C, Lallas A et al A prospective open‐label clinical trial of efficacy of the every week administration of adalimumab in the treatment of hidradenitis suppurativa. J Drugs Dermatol 2012; 11: s15–s20. [PubMed] [Google Scholar]
- 69. Zouboulis C. Adalimumab efficacy in hidradenitis suppurativa patients is sustained at least three years with weekly dosing: results from a phase 3 open‐label extension study (PIONEER). J Am Acad Dermatol 2017; 76: 33.28029390 [Google Scholar]
- 70. Armyra K, Kouris A, Markantoni V, Katsambas A, Kontochristopoulos G. Hidradenitis suppurativa treated with tetracycline in combination with colchicine: a prospective series of 20 patients. Int J Dermatol 2017; 56: 346–350. [DOI] [PubMed] [Google Scholar]
- 71. Fadel MA, Tawfik AA. New topical photodynamic therapy for treatment of hidradenitis suppurativa using methylene blue niosomal gel: a single‐blind, randomized, comparative study. Clin Exp Dermatol 2015; 40: 116–122. [DOI] [PubMed] [Google Scholar]
- 72. Tierney E, Mahmoud BH, Hexsel C, Ozog D, Hamzavi I. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium‐doped yttrium aluminium garnet laser. Dermatol Surg 2009; 35: 1188–1198. [DOI] [PubMed] [Google Scholar]
- 73. Zouboulis CC, Tzellos T, Kyrgidis A et al Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017; 177: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 74. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014; 20: 953–966. [DOI] [PubMed] [Google Scholar]
- 75. Greenberg JD, Kremer JM, Curtis JR et al Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 576–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the HS ALLIANCE national faculties.
Appendix S2. Search strings and PRISMA diagrams for each clinical question literature search.
Appendix S3. Comparison of current recommendations with existing clinical practice guidelines and JAMA rating of guidelines.


