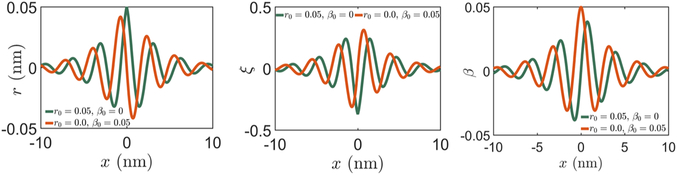
Figure 4.
Variation of r, k3, ξ, and β+ = β− = β for a single protein. The red curve corresponds to the boundary conditions β0 = 0, r0 = 0.05 nm, and the green curve to r0 = 0, β0 = 0.05. Even if r0 = 0, i.e., the protein does not change the radius of the molecule at the binding site, a change in the phase angle β0 ≠ 0 can cause the radius to change at locations away from the binding site. Similar coupling exists among other strain parameters. The magnitudes r0 and β0 chosen here ensure that the change in the groove width, a parameter whose magnitude is known,12 is in the correct range (3 Å). The decay length is ld = ζ−1 ≈ 10 bp, which is close to the one documented in literature.7,23