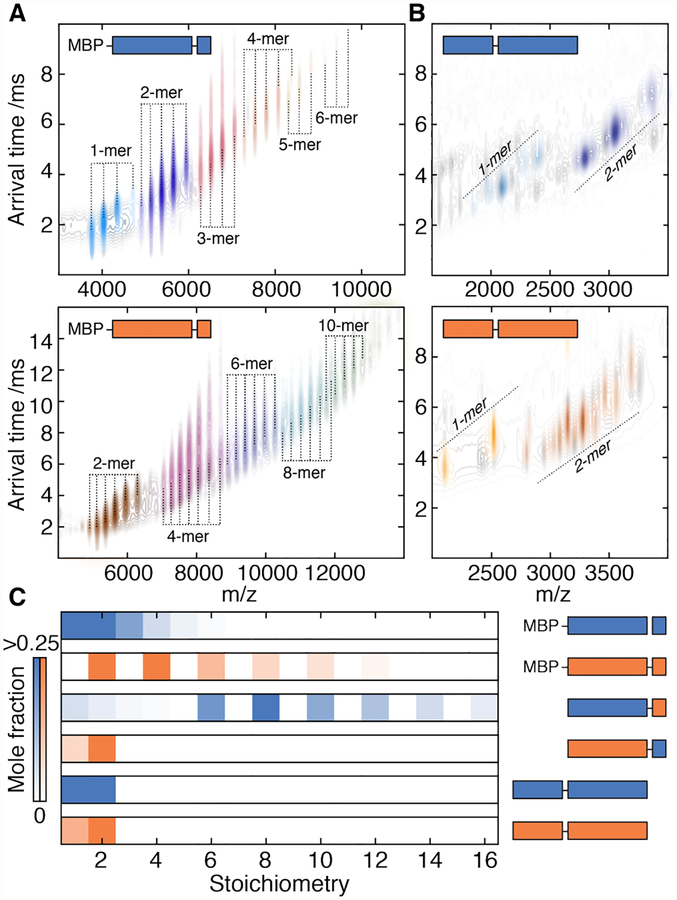
Figure 2. Oligomeric interfaces form in a hierarchical order.
A) IM-MS spectra of truncated constructs of WT-1 (upper) and WT-2 (lower) lacking the N-terminal region. The two dimensions of separation (m/z and arrival time, which depends on collision cross-section) separate charge-state series corresponding to a series of stoichiometries (coloured individually). Both truncated proteins assemble into polydisperse ensembles. MPB – maltose binding protein.
B) IM-MS spectra of truncated constructs of WT-1 (upper) and WT-2 (lower) lacking the C-terminal tail. Both proteins do not assemble beyond dimers. Truncations on the exposed N-terminus result in several charge-series for monomers and dimers that are separated in the arrival time dimension (see Fig. S8 for detailed assignments).
C) Distribution of stoichiometries populated by truncated constructs extracted from spectra in A, B, Fig S6. The C-terminal tail is required for assembly beyond dimers, whereas the N-terminus is required for monodisperse 12-mers. The α2C1 construct (Fig. S8E) does not oligomerize, indicating an unfavourable α·C interaction.