Figure 1.
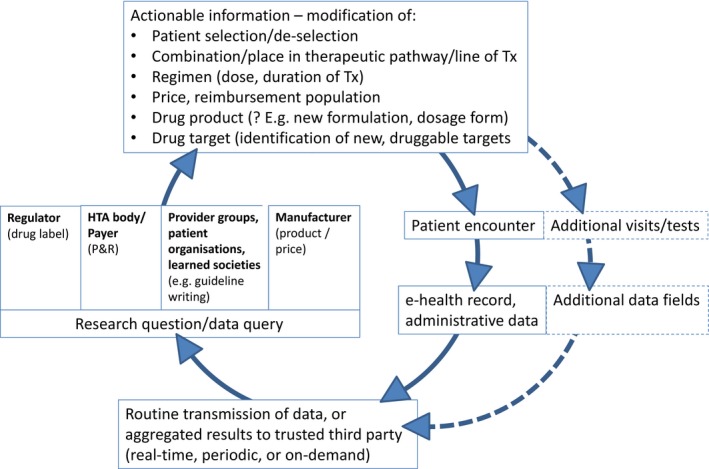
The learning healthcare system applied to pharmaceuticals. The graph depicts the vision of repeated cycles of “learn and improve” that are expected to make the drug development and use chain more effective and efficient. The clockwise cycle starts at the right‐hand side with a patient encountering a healthcare professional. This could happen in a hospital, office, or any other setting; the encounter will generate entries in the patient's eHR, electronic prescribing system, and/or other administrative databases that are transmitted to a third party (bottom of graph). We assume that a third party may likely be necessary in many healthcare systems to ensure trust in the overall process, but specifically to guarantee patient data protection and anonymity as well as gatekeeper and custodian functions and, where needed, to ensure linkage across different data sources (e.g., linking hospital and primary care data). Where individual patient data cannot be transmitted and need to remain behind local firewalls (e.g., for legal reasons), a distributed data approach will be required. In such cases, the third party could act as a conduit to distribute queries in the form of standardized computer programs to local database owners who run the queries and return aggregated results to the third party (“share answers not data”). Depending on the country and healthcare system, the third party may be a new dedicated organization, a national regulator or health service, or another existing entity. Following agreed procedures, data or aggregated results will be made available to vested stakeholders to answer research questions or other data queries (left‐hand side of graph). Relevant research questions may be raised by a drug regulator and relate to on‐market benefit–risk assessment in various patient populations or by HTA bodies/healthcare payers about on‐market drug use, value, relative effectiveness, cost‐effectiveness, coverage, or pay‐for‐performance and risk‐sharing schemes. Alternatively, provider groups, patient organizations, or learned societies could initiate data queries for research projects or to inform clinical guideline development. Last, the data (or aggregated results) can be used by drug manufacturers for postlicensing monitoring, risk management, or even postlicensing or de novo drug development. The kind of actionable information that could be generated from the data is illustrated by examples at the top of the graph: information on favorable or unfavorable treatment outcomes might help select or deselect patients with defined (phenotypic or genotypic) characteristics; in turn, this will be reflected in an optimized treatment‐eligible population in the regulatory label and for payer coverage; the information can also inform the value assessment, providing a basis for price negotiations; results of secondary data analysis may provide guidance on the best use of a drug, e.g., on best combinations, place in the therapeutic pathway (e.g., second‐ vs. first‐line treatment), start–stop criteria for treatment or dose selection; perhaps less frequently, information may be translated into new drug dosage forms or formulations (e.g., for special populations, like elderly people) or support hypothesis generation on novel treatment targets. Further downstream (right‐hand side of graph, dotted lines), the information may trigger a change of the routine practice of medicine, i.e., of future patient encounters: additional patient visits (or longer intervals), the incorporation of new or different tests, and, ultimately, the modification of the eHR itself (by way of adding new data fields). At that stage, the learning healthcare system would have achieved its goal and a new cycle of “learn and improve” starts. As a drug moves along its developmental and on‐market phases, the nature of research questions asked is expected to change. Although the focus during the R&D phase is expected to be on, e.g., quantification of unmet medical need, description of the natural history of a patient (sub‐) population, or elucidation of standard of care, the focus during the on‐market phase is likely on optimization of the treatment population, quantification of effectiveness in clinical practice, and safety, along with available therapeutic options. The scope of RWD‐based knowledge generation is broader than classical “phase IV” studies. eHR, electronic health record; HTA, health technology assessment; P&R, pricing and reimbursement; R&D, research and development. RWD, real‐world data; Tx, treatment.
