Abstract
Introduction
To objectively quantify patients' physical activity and analyze the relationships between physical activity levels, psychopathology, and sedative medication in acute hospital dementia care.
Materials and Methods
In this cross‐sectional study, we assessed the patients' physical activity based on data collection by hybrid motion sensors attached on their lower back. Daily doses of antipsychotics have been converted to olanzapine‐equivalents and daily benzodiazepine medication is reported as diazepam‐equivalents. We assessed patients' neuropsychiatric symptoms with the Neuropsychiatric Inventory and the Cohen‐Mansfield Agitation Inventory.
Results
We analyzed motion sensor data from 64 patients (MMSE M = 18.6). On average, patients were lying for 11.5 hours, sitting/standing sedentary for 10.3 hours, sitting/standing active for 1.0 hours, and walking for 1.2 hours per day. The analysis revealed no correlations between patients' physical activity and antipsychotic or benzodiazepine medication. More severe neuropsychiatric symptoms were associated with a decrease in the patients' physical activity (r = .32, P = .01). In particular, patients with apathy symptoms were less physically active than patients without apathy symptoms.
Discussion
The results reveal that most of the patients in acute dementia care had very low levels of physical activity. Their physical inactivity may be due to the severity of their neuropsychiatric symptoms, especially apathy. Antipsychotic and benzodiazepine medication appeared to have less impact on patients' physical activity. Dementia care should pay more attention to prevent physical inactivity in patients.
Keywords: antipsychotics, benzodiazepines, body‐worn motion sensors, dementia, hospital dementia care, physical activity
Key points.
Patients in acute dementia care show an unexpected low level of physical activity
Psychopathological symptoms—especially apathy—are related to the low level of physical inactivity, not the dose of sedative medication
Dementia care should pay more attention to prevent physical inactivity in patients
1. INTRODUCTION
Dementia is one of the biggest global health care challenges of our time. In 2016, there were more than 47 million people suffering from dementia, and this number will increase to more than 131 million by 2050.1 Alongside the cognitive impairment, neuropsychiatric signs and symptoms (NPS) in dementia are a big challenge for clinical psychiatry and lead to a high caregiver burden.2 During the course of the disease, almost every patient suffers from NPS.3 The term NPS covers psychological symptoms such as delusions, hallucinations, depression, sleeplessness and anxiety, and behavioral symptoms such as physical aggression, wandering, and restlessness.4 Periods of exacerbated NPS often lead to hospital admission and early institutionalization.5
Pharmacological and non‐pharmacological treatment of NPS is one of the key challenges in dementia care. Non‐pharmacological treatments are generally the first‐line treatments for NPS, eg, staff training in NPS management strategies, recreational activities, music therapy, or other forms of sensory stimulation.6 Recent reviews indicate that physical exercise interventions have a positive impact on NPS.7, 8 The pharmacological treatments for NPS are antipsychotics, anticonvulsants, antidepressants, anxiolytics, benzodiazepines, cholinesterase inhibitors, and NMDA modulators.9
There is growing evidence of a link between dementia patients' physical activity level and NPS.10 Christofoletti et al11 found that more sedentary patients exhibited more NPS, which raises the issue of the impact of antipsychotic and benzodiazepine medication on the physical activity levels of patients in acute dementia care.
We aimed to take advantage of the increasing use of technology in geriatric psychiatry12 to quantify acute dementia patients' physical activity objectively and analyze the relationship between patients' physical activity levels, psychopathology, and sedative medication.
2. MATERIALS AND INSTRUMENTS
2.1. Study design
We conducted a cross‐sectional investigation in the Department of Geriatric Psychiatry of the LVR‐Hospital in Cologne as part of a randomized clinical trial.13 This analysis includes the baseline measurements of all patients before randomization. The Ethics Commission of the German Sport University Cologne and the Ethics Commission of the North‐Rhine Medical Chamber approved the study (reference number: 2014216). German National Register of Clinical Trials: DRKS00006740, date of registration: 28.10.2014).
2.2. Sample
The sample comprised patients from two closed dementia wards. The inclusion criteria were (1) diagnosis of dementia according to ICD‐10‐criteria,14 (2) ability to perform the timed up and Go test (TuG)15 without assistance, and (3) written informed consent from the patient, if the patient had a score of ≥20 in the Mini Mental Status Examination (MMSE)16 and was able to rephrase the aims and content of the study in his own words; in patients with a MMSE <20, written consent from the patient and written informed consent from his or her legal guardian was required. The exclusion criteria were diagnosis of symptomatic, non‐vascular or non‐neurodegenerative dementia, diagnosis of delirium, and no legal guardian. In total 87 patients were recruited for the umbrella RCT. The baseline assessment results of this sample have been included in this analysis.
2.3. Data acquisition
The assessment of the patients' physical activity was based on a 72‐hour period of data acquisition by a hybrid motion sensor (uSense) fixed on the patients' lower back. Based on these recordings, we analyzed patients' physical activity over a 24‐hour period from 00:00 and 24:00, using 1‐second time bins. The uSense wearable sensor and its software for signal processing and activity recognition are an outcome of the FARSEEING EU project. The software allows quantitative data analysis, and it has already been used in patients with dementia,17 in older people residing in independent‐living retirement homes,18 and in community‐dwelling older adults.19 The activity recognition software is able to identify four activity states: lying; sedentary, either sitting or standing; active, either sitting or standing; and walking. In addition, daily step counts were analyzed. For this trial, we analyzed dementia patients' doses of antipsychotics and benzodiazepines for the 24‐hour period in which the activity assessment was carried out. Dose of antipsychotics was converted into the olanzapine‐equivalent dose per day.20 Dose of benzodiazepine medication was converted into the diazepam‐equivalent dose per day.21
We used MMSE,16 Demtect,22 and Clock Drawing test23 to assess cognitive impairment. Information about cognitive reserve was also captured.24 The Bayer Activities of Daily Living scale was used to estimate patients' ability to perform everyday activities.25 The TuG15 and the 10‐m gait speed test were used to measure patients' mobility.25 Neuropsychiatric symptoms were assessed using the Neuropsychiatric Inventory (NPI)27 and the Cohen‐Mansfield Agitation Inventory (CMAI)28 was used to rate the patients' agitation symptoms. All outcome measurements were conducted by experienced assessors from nursing and medical staff.
2.4. Statistical analysis
All statistical analyses were performed using SPSS Version 24.0.29 Outliers in the dependent variables (OPZ, DPZ, physical activity, CMAI, NPI) were excluded by the 1.5‐fold inter‐quartile from the upper or lower box plot quartile. We analyzed differences between the two medication groups (use of antipsychotics and/or benzodiazepines vs no medication) with Chi‐squared tests in the case of categorical variables (sex, dementia diagnosis), Mann‐Whitney U‐test in the case of ordinal variables (NPI, CMAI, Bayer Activities of Daily Living), unpaired t‐tests in the case of normally distributed continuous variables (body mass index, MMSE, Demtect, clock‐drawing test, TUG, 10‐m gait speed, physical activity levels), and Mann‐Whitney U‐tests in the case of nonparametric continuous variables. We used Pearson's correlations to assess of the relationship between use of antipsychotics and benzodiazepines and patient's physical activity.
Correlation ranges of .70 to .90 were regarded as high, ranges of .50 to .70 as moderate, and ranges of .30 to .50 as low.30 A significance level of α = .05 was used for all tests.
3. RESULTS
Eighty‐seven patients were recruited for this trial. Initially, 86 patients accepted the uSense sensor attachment. One patient refused the sensor attachment at the beginning. Five patients removed the device during the 72‐hour recording period. In the raw data analysis, we discovered that four data sets were incomplete or missing. One patient who moved with the help of a four‐wheeled walker was excluded from the analysis of gait time and total steps per day because we were unable to detect her steps accurately. Correction for outliers eliminated n = 7 patients due to their physical activity and n = 6 patients due to the applied sedative medication. No patient was excluded due to the NPS. Out of the N = 87 recruited patients, n = 64 patients with a synchronized 24‐hour sensor‐recording were included in the analysis. The group's characteristics are presented in Table 1.
Table 1.
Patient characteristics (N = 64; female n = 30 [47%])
| M | SD | Min. | Max. | N (%) | |
|---|---|---|---|---|---|
| Age | 81 | 6.2 | 67 | 95 | 64 |
| Body mass index [kg/m2] | 25.6 | 4.1 | 18 | 34 | 64 |
| Diagnosis | |||||
| Alzheimer's disease dementia | 17 (27) | ||||
| Vascular dementia | 10 (16) | ||||
| Dementia, mixed type | 34 (53) | ||||
| Parkinson's disease dementia | 2 (3) | ||||
| Lewy‐body dementia | 1 (1) | ||||
| Mini‐mental status examination | 18.6 | 5.5 | 7 | 27 | 64 |
| Demtect | 4.2 | 1.6 | 1 | 6 | 58 |
| Clock‐drawing test | 5.0 | 3.4 | 0 | 14 | 57 |
| Neuropsychiatric inventory (NPI) | 19.2 | 12.2 | 0 | 51 | 64 |
| Cohen‐Mansfield agitation inventory (CMAI) | 49.1 | 12.0 | 29 | 83 | 64 |
| Cognitive reserve capacity, years of education | 11.9 | 2.6 | 7 | 18 | 63 |
| Bayer‐activities of daily living | 7.7 | 1.6 | 2.3 | 9.7 | 64 |
| Timed up and go test [s] | 14.4 | 5.2 | 7.3 | 32.9 | 64 |
| 10 meter gait speed [m/s] | 0.8 | 0.2 | 0.3 | 1.6 | 64 |
| Benzodiazepine dose (DED) [mg/day] | 4.0 | 1.9 | 0.3 | 7.5 | 10 (16) |
| Antipsychotic dose [mg/day] | 2.2 | 1.4 | 0.3 | 5.7 | 48 (75) |
| Antipsychotics only | 39 (61) | ||||
| Only benzodiazepines | 1 (2) | ||||
| Benzodiazepines and antipsychotics | 9 (14) | ||||
| No benzodiazepines or antipsychotics | 15 (23) | ||||
M = mean; SD = standard deviation; Min. = minimum; Max. = maximum; Range and scaling of psychopathometric instruments: NPI: 0‐144 points (0 meaning no symptoms); CMAI: 29‐203 points (29 meaning no symptoms); DED = Diazepam equivalent dose; OED = Olanzapine equivalent dose.
Most of the patients (53%) suffered from mixed type of dementia. All patients completed the MMSE, and the mean MMSE score was 18.6 points. Not all patients performed the Demtect (N = 58) and Clock drawing test (N = 57) because some of them had problems understanding the instructions. Forty‐eight of the 64 patients received antipsychotic medication, and 10 patients received benzodiazepine medication. We did not find any differences regarding patient characteristics, neuropsychiatric symptoms, and OPZ/DPZ between the n = 64 included and the n = 10 patients, who refused or had incomplete data sets and the n = 13 outliers. The neuropsychiatric symptoms of the sample are shown in Table 2.
Table 2.
Psychopathological symptoms in terms of the neuropsychiatric inventory (NPI) domains (N = 64)
| NPI Domains | n (%) | M | SD | Min. | Max. |
|---|---|---|---|---|---|
| Delusions | 22 (34) | 6.5 | 3.0 | 2 | 12 |
| Hallucinations | 10 (16) | 7.1 | 4.0 | 2 | 12 |
| Agitation/aggression | 38 (59) | 5.7 | 2.8 | 2 | 12 |
| Depression/dysphoria | 33 (52) | 4.8 | 3.0 | 1 | 12 |
| Anxiety | 18 (28) | 5.8 | 2.8 | 2 | 12 |
| Elation/euphoria | 3 (5) | 4.7 | 3.1 | 2 | 8 |
| Apathy/indifference | 29 (45) | 4.7 | 2.1 | 1 | 8 |
| Disinhibition | 10 (16) | 4.9 | 3.0 | 3 | 12 |
| Irritability/lability | 36 (56) | 5.1 | 2.4 | 2 | 12 |
| Aberrant motor behavior | 30 (47) | 5.1 | 1.9 | 3 | 8 |
| Sleep and night‐time behavior disorders | 12 (19) | 6.1 | 1.9 | 3 | 9 |
| Appetite/eating changes | 3 (5) | 10.7 | 2.3 | 8 | 12 |
Patients symptoms; frequency*severity scores (0‐12; 0 indicates absent); M = mean; SD = standard deviation; Min. = minimum; Max. = minimum.
The most common symptoms in the final sample (N = 64) were agitation and aggression (59%), depression and dysphoria (52%), and irritability and lability (56%). Means for physical activity parameters, based on the sensor data, are shown in Table 3.
Table 3.
Sensor‐based means for physical activity parameters (N = 64; N = 63 for “gait” and “steps” [see text for further explanation])
| N | M | SD | Min. | Max. | |
|---|---|---|---|---|---|
| Posture/activity | |||||
| Lying, h/day (%) | 64 | 11.5 (48) | 2.1 (9) | 6.5 (27) | 16.0 (67) |
| Sedentary sitting/standing, h/day (%) | 64 | 10.3 (43) | 2.1 (9) | 6.0 (25) | 15.3 (64) |
| Active sitting/standing, h/day (%) | 64 | 1.0 (4) | 0.4 (2) | 0.3 (1) | 2.1 (9) |
| Gait, h/day (%) | 63 | 1.2 (5) | 0.6 (3) | 0.2 (1) | 2.6 (11) |
| Total steps per day | 63 | 6193 | 3204 | 796 | 13 885 |
M = mean; SD = standard deviation; Min. = minimum; Max. = maximum.
Patients' daily number of steps ranged from 796 to 13 885, and the mean number of steps per day was 6193 (SD = 3204). The mean duration of physical inactivity (sum of daily hours of lying and sedentary sitting/standing) was 21 hours 50 minutes, representing 91% of the day (Figure 1). Duration of physical inactivity ranged from 19 hours 20 minutes to 23 hours 12 minutes per day.
Figure 1.
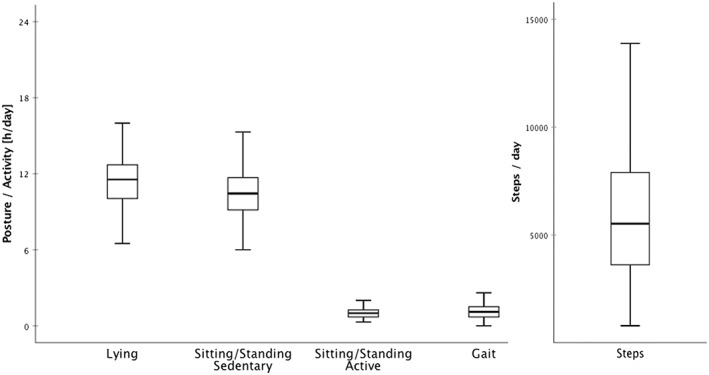
Sensor‐derived physical activity data [mean total hours/posture/day] (N = 64; N = 63 for “gait” and “steps” [see text for further explanation])
Correlations between patients' physical activity and their doses of antipsychotic and benzodiazepine medication are shown in Table 4.
Table 4.
Correlations between patients' physical activity and benzodiazepine medication (diazepam‐equivalent dose; DED) and antipsychotic medication (olanzapine‐equivalent dose; OED)
| n | Lying, h/day | Sed, h/day | Act, h/day | n | Gait, h/day | Steps/day | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |||
| DED | 10 | 0.25 | 0.50 | −0.20 | 0.57 | 0.21 | 0.57 | 9 | −0.32 | 0.40 | −0.19 | 0.63 |
| OED | 48 | −0.01 | 0.93 | 0.11 | 0.46 | −0.19 | 0.19 | 47 | −0.13 | 0.75 | −0.16 | 0.70 |
Sed = sedentary sitting/standing; Act = active sitting/standing; steps = total steps per day; r = Pearson correlation coefficient; Note: Gait and steps/day: one patient had to be excluded—see text for further explanation.
The analysis revealed no correlations between physical activity and dose of antipsychotics or benzodiazepines. Physical inactivity was not correlated with diazepam‐equivalent dose (r = .09, P = .46) or olanzapine‐equivalent dose (r = .20, P = .11). Compared with the group of patients who only received antipsychotics (n = 39), the group receiving both antipsychotic and benzodiazepine medication (n = 9) spent more time lying down (z = −2.31, P = 0.02). Physical activity was similar in the group of patients not taking either kind of medication (n = 15) and the nine patients receiving both, antipsychotics and benzodiazepines, eg, for lying h/day (z = −1.73, P = 0.08) or steps per day (z = −0.09, P = 0.95).
Total NPI score shows a moderate positively correlation to the patients' physical inactivity (r = .32, P = .01). Psychopathological symptoms expressed as NPI score were negatively associated physical activity. More severe psychopathological symptoms as rated with the NPI are associated with less physical activity. There were n = 29 patients (45%) suffering from apathy symptoms, and the mean duration of physical inactivity in this subgroup was 22 hours 5 minutes/day (SD = 4). On average n = 35 patients (56%) without apathy symptoms were active for 27 minutes more per day than patients with apathy symptoms (z = −2.12, P = 0.34). Total CMAI score was not correlated with physical inactivity (r = −0.21, P = .11).
4. DISCUSSION
The aim of this study was to assess overall physical activity level in a large cohort of patients in acute dementia care. We also investigated the relationships between physical activity and psychopathological symptoms and use of antipsychotic and benzodiazepine medication in patients receiving acute dementia care.
The results revealed that most patients in acute dementia care had very low levels of physical activity. The NPI showed a positive correlation to the patients' physical inactivity, with more severe psychopathological symptoms related to a lower amount of physical activity. Overall, patients' level of physical inactivity does not seem to be associated with their dosage of antipsychotic or benzodiazepine medication, but the small group of patients receiving both antipsychotics and benzodiazepines (n = 9, 14%) were sedentary for a greater proportion of the day than other patients. This might be due to receipt of benzodiazepine medication in addition to antipsychotics. Alternatively, more severe psychopathology may be responsible for the low level of physical activity. Our results reveal that psychopathological symptoms, as measured by the NPI, play an important role in explaining physical inactivity in most of our patients. In particular, we found a low correlation of the NPI score with the patients' physical inactivity and that apathy symptoms, present in 29 patients (45%) patients, were linked to lower physical activity; mean duration of physical activity in patients showing apathy was 27 minutes per day less than in the 35 patients (55%) who did not show apathy.
Our patients were physically inactive for more than 90% of the day, eg, some patients were inactive for more than 23 hours per day. Similar low levels of physical activity and correlations to NPS in dementia care have been reported by Christofoletti et al,11 and studies in which wrist‐worn actigraphic devices were used to assess patients' physical activity.31, 32, 33 Unlike wrist actigraphy, using hybrid motion sensors attached to the lower back, as we did in this trial, allows to analyze mobility‐related physical activity rather than just upper limb movement.
Using motion sensors allows patients' physical activity to be quantified objectively. The sensor data indicate that our sample was sedentary and physically inactive for most of the day (Figure 1), eg, some patients only get up from bed or stand up from a chair to go to the toilet or take meals. The use of objective measures of the patients' physical activity puts a focus on one of the major problems in dementia care: patients' physical inactivity. In acute dementia care settings, patients with particularly severe or disturbing NPS and those suffering from aberrant motor behavior are often more visible on the wards as they tend to require more medical and nursing resources. However, as our analysis has shown, the majority of patients was physically inactive and sedentary, and we assume from our clinical experience, that sedentary patients often go more or less unnoticed in acute dementia care. From the clinical expertise of the authors, this phenomenon can very often be translated to nursing‐home or home‐care settings. Furthermore, this study confirms the findings of Eggermont and Scherder,34 who found that nursing home residents suffering from dementia were classified as ambulatory, were nevertheless sedentary for almost the whole day. This physical inactivity may be directly linked to the development and exacerbation of psychopathological symptoms.10, 11
The availability of objective, quantitative data on dementia patients' physical inactivity could be used to prompt a change in care strategy and a new focus on physical activity in acute dementia care. There is increasing evidence that exercise programs have a beneficial effect on patients' ability to perform activities of daily living as well as decreasing the caregiver burden in dementia care.35 Furthermore, structured physical exercise interventions have been shown to be an effective treatment for NPS in dementia.7, 8
It is important to note that our analyses did not include all medications that could influence patients' physical activity, for example we did not assess use of antidepressants and antidementics. Because we wanted to analyze synchronized activity and medication data, only sensor data from the first complete 24‐hour period from midnight were used in the analyses presented here. Recording activity over a longer period would allow a more robust analysis and interpretation of patients' physical activity. Because we excluded outliers our analysis does not cover the full spectrum of physical activity in dementia patients, eg, a patient who showed a wandering phenomenon and thus took more than 40 000 steps per day had to be excluded.
These limitations should be taken into account when interpreting the results. As objective assessments of dementia patients' physical activity become more widespread, we may see an increase in the emphasis on motor behavior. The availability of objective measures of physical activity makes it possible to design and evaluate individualized exercise interventions that might help to reduce NPS and increase patients, caregivers', and medical professionals' quality of life.
In summary, physical inactivity in patients suffering from dementia seems to be a feature of the psychopathology of the disease rather than an effect of antipsychotic or benzodiazepine medication. Dementia care should pay more attention to prevent physical inactivity in patients. There is an urgent need for effective non‐pharmacological treatments for NPS as use of antipsychotics and benzodiazepines to treat NPS in patients suffering from dementia has been linked to serious adverse effects.36, 37
AUTHOR'S CONTRIBUTION
T.F. and M.G.: study concept, data acquisition, statistical analysis, interpretation of data, draft and revision of the manuscript. S.G. and S.M.: analysis of the sensor raw data, revision of the manuscript. W.Z.: study concept, further data analysis, interpretation of data, draft and revision of the manuscript. P.H.: study concept, data acquisition, clinical interpretation of data, draft, and revision of the manuscript.
SPONSOR'S ROLE
This investigation was partly funded by a young researcher grant from the German Sport University Cologne (T.F.), German Research Foundation (DFG) SFB 654, Teilprojekt 14 (P.H.), FARSEEING FP7/2007‐2013, grant agreement n. 288940 (S.M. and W.Z.), Alzheimer Forschung Initiative e. V. (S.G.), and the institutional budgets of the authors. The funding agencies did not play any active role in the scientific investigation and reporting of the study. All authors had complete independence in this study.
CONFLICTS OF INTERESTS
None declared.
ACKNOWLEDGEMENTS
The authors thank all patients and legal guardians. We also thank the board of the directors of the LVR‐hospital as well as the staff at the LVR‐Hospital Cologne, for their support during the study. Furthermore, the authors acknowledge Hartmut Reinbold, Gela Utzerath, and Dirk Reske for advice on equivalent dosage calculation and Ingrid Becker for supporting the statistical analyses.
Fleiner T, Gersie M, Ghosh S, Mellone S, Zijlstra W, Haussermann P. Prominent physical inactivity in acute dementia care: Psychopathology seems to be more important than the dose of sedative medication. Int J Geriatr Psychiatry. 2019;34:308–314. 10.1002/gps.5021
REFERENCES
- 1. Prince M, Comas‐Herrera MA, Knapp M, et al. World Alzheimer Report 2016 improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London, Alzheimer's Disease International, 2016. https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf. Accessed March 21, 2018
- 2. Reed C, Belger M, Dell'Agnello G, et al. Caregiver burden in Alzheimer's disease: differential associations in adult‐child and spousal caregivers in the GERAS observational study. Dement Geriatr Cogn Dis Extra. 2014;4(1):51‐64. 10.1159/000358234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475‐1483. http://www.ncbi.nlm.nih.gov/pubmed/12243634 [DOI] [PubMed] [Google Scholar]
- 4. Draper B, Finkel SI, Tune L. An introduction to BPSD In: Draper B, Brodaty H, Finkel SI, eds. The IPA Complete Guides to Behavioral and Psychological Symptoms of Dementia. Specialists Guide. Northfield, IL: International Psychogeriatric Association (IPA); 2016:1‐16. [Google Scholar]
- 5. Steele C, Rovner B, Chase G, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer's disease. Am J Psychiatry. 1990;147(8):1049‐1051. 10.1176/ajp.147.8.1049 [DOI] [PubMed] [Google Scholar]
- 6. Fairbairn A, Gould N, Kendall T. Dementia: Supporting People with Dementia and Their Carers in Health and Social Care—Clinical Guideline [CG42]. National Institute for Health and Care Excellence: Manchester, UK; 2006. www.guidance.nice.org.uk/cg42. [Google Scholar]
- 7. Souto Barreto P, Demougeot L, Pillard F, et al. Exercise training for managing behavioral and psychological symptoms in people with dementia: a systematic review and meta‐analysis. Ageing Res Rev. 2015;24(B):274‐285. 10.1016/j.arr.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Fleiner T, Leucht S, Förstl H, Zijlstra W, Haussermann P. Effects of short‐term exercise interventions on behavioral and psychological symptoms in patients with dementia: a systematic review. J Alzheimers Dis. 2017;55(4):1583‐1594. 10.3233/JAD-160683 [DOI] [PubMed] [Google Scholar]
- 9. Ballard C, Day S, Sharp S, Wing G, Sorensen S. Neuropsychiatric symptoms in dementia: importance and treatment considerations. Int Rev Psychiatry. 2008;20(4):396‐404. 10.1080/09540260802099968 [DOI] [PubMed] [Google Scholar]
- 10. Scherder EJA, Bogen T, Eggermont LHP, Hamers JPH, Swaab DF. The more physical inactivity, the more agitation in dementia. Int Psychogeriatr. 2010;22(8):1203‐1208. [DOI] [PubMed] [Google Scholar]
- 11. Christofoletti G, Oliani MM, Bucken‐Gobbi LT, Gobbi S, Beinotti F, Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics. 2011;66(4):613‐618. 10.1590/S1807-59322011000400015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vahia IV, Ressler KJ. Beyond the buzz. Am J Geriatr Psychiatry. 2017;25(8):815‐818. 10.1016/j.jagp.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 13. Fleiner T, Zijlstra W, Dauth H, Haussermann P. Evaluation of a hospital‐based day‐structuring exercise programme on exacerbated behavioural and psychological symptoms in dementia—the exercise carrousel: study protocol for a randomised controlled trial. Trials. 2015;16(1). 10.1186/s13063-015-0758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision. Chapter V: Mental and behavioural disorders (F00‐F99). Geneva: World Health Organization; 2016. [Google Scholar]
- 15. Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142‐148. [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 17. Fleiner T, Haussermann P, Mellone S, Zijlstra W. Sensor‐based assessment of mobility‐related behavior in dementia: feasibility and relevance in a hospital context. Int Psychogeriatr. 2016;28(10):1687‐1694. 10.1017/S1041610216001034 [DOI] [PubMed] [Google Scholar]
- 18. Chigateri N, Kerse N, MacDonald B, Klenk J. Validation of walking episode recognition in supervised and free‐living conditions using triaxial accelerometers. In: Proceedings of the 2017 World Congress of International Society for Posture & Gait Research, Fort Lauderdale, Florida, US: 25–29 June 2017; 2017; 289–290. https://www.ispgr.org/cpages/florida‐2017.
- 19. Leach JM, Mellone S, Palumbo P, Bandinelli S, Chiari L. Natural turn measures predict recurrent falls in community‐dwelling older adults: a longitudinal cohort study. Sci Rep. 2018;8(1):4316 10.1038/s41598-018-22492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686‐693. 10.1176/appi.ajp.2009.09060802 [DOI] [PubMed] [Google Scholar]
- 21. Holzbach R, Martens MS, Kalke J, Raschke P. Zusammenhang zwischen Verschreibungsverhalten der Arzte und Medikamentenabhängigkeit ihrer Patienten. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2010;53(4):319‐325. 10.1007/s00103-010-1029-8 [DOI] [PubMed] [Google Scholar]
- 22. Kalbe E, Kessler J, Calabrese P, et al. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19(2):136‐143. 10.1002/gps.1042 [DOI] [PubMed] [Google Scholar]
- 23. Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725‐729. [DOI] [PubMed] [Google Scholar]
- 24. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448‐460. [PubMed] [Google Scholar]
- 25. Hindmarch I, Lehfeld H, Jongh P, Erzigkeit H. The Bayer Activities of Daily Living Scale (B‐ADL). Dement Geriatr Cogn Disord. 1998;9(S2):20‐26. [DOI] [PubMed] [Google Scholar]
- 26. Ries JD, Echternach LN, Gagon Blodgett M. Test‐retest reliability and minimal detectable change scores for the timed “up & go” test, the six‐minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569‐579. 10.2522/ptj.20080258 [DOI] [PubMed] [Google Scholar]
- 27. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurol. 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
- 28. Cohen‐Mansfield J. Instruction Manual for the Cohen‐Mansfield Agitation Inventory (CMAI). Rockville, Maryland: The Research Institute of the Hebrew Home of Greater Washington; 1991. [Google Scholar]
- 29. SPSS Statistics [computer program]. Version 24. https://www‐01.ibm.com/software/de/stats24/. 2017.
- 30. Hinkle D, Wiersma W, Jurs S. Applied Statistics for the Behavioral Sciences. 5th ed. Boston: Houghton Mifflin; 2003. [Google Scholar]
- 31. Kuhlmei A, Walther B, Becker T, Müller U, Nikolaus T. Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy. Eur Psychiatry. 2013;28(2):94‐97. 10.1016/j.eurpsy.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 32. Valembois L, Oasi C, Pariel S, Jarzebowski W, Lafuente‐Lafuente C, Belmin J. Wrist actigraphy: a simple way to record motor activity in elderly patients with dementia and apathy or aberrant motor behavior. J Nutr Health Aging. 2015;19(7):759‐764. 10.1007/s12603-015-0530-z [DOI] [PubMed] [Google Scholar]
- 33. David R, Rivet A, Robert PH, et al. Ambulatory actigraphy correlates with apathy in mild Alzheimer's disease. Dementia. 2010;9(4):509‐516. 10.1177/1471301210381678 [DOI] [Google Scholar]
- 34. Eggermont LHP, Scherder EJA. Ambulatory but sedentary: impact on cognition and the rest‐activity rhythm in nursing home residents with dementia. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):P279‐P287. http://www.ncbi.nlm.nih.gov/pubmed/18818442 [DOI] [PubMed] [Google Scholar]
- 35. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S, Cochrane Dementia and Cognitive Improvement Group . Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4:CD006489 10.1002/14651858.CD006489.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. US Food and Drug Administration . Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances, 2005. http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 26, 2016
- 37. Tamblyn R, Abrahamowicz M, du Berger R, et al. A 5‐year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc. 2005;53(2):233‐241. 10.1111/j.1532-5415.2005.53108.x [DOI] [PubMed] [Google Scholar]


