Figure 2.
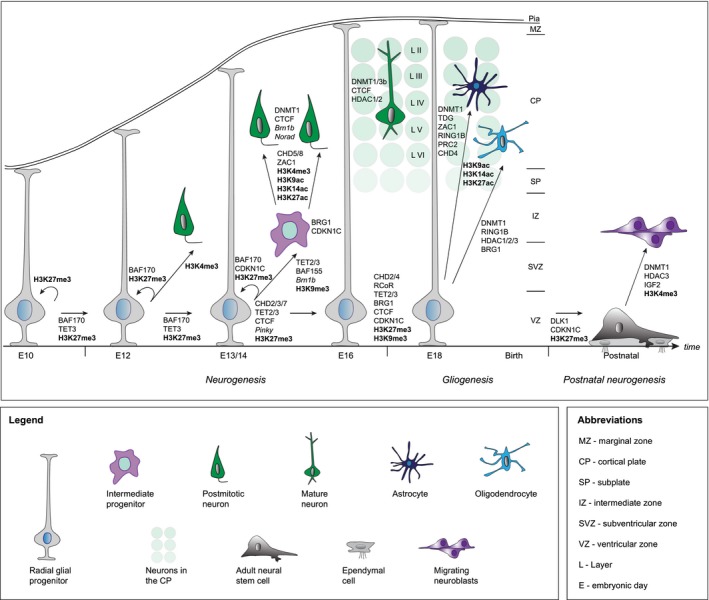
Epigenetic factors and modifications regulating the generation of cell‐type diversity during RGP lineage progression. High content of repressive H3K9me2/me3 and H3K27me3 marks and the presence of chromatin regulators such as BAF170, BRG1, CHD2, Pnky, TET1/3 and CTCF regulate stem cell maintenance and RGP self‐renewal while suppressing genes involved in neuron differentiation. For neuron production, repressive marks are replaced by active marks such as H3K4me3 or histone acetylation to promote expression of proneural genes mediating neuronal differentiation and maturation. Transition from repressive to activating epigenetic regulation is mediated through BAF155, BRN1, CHD5/8 and CBP. PRC and histone acetylation are essential for mediating the neurogenic to gliogenic transition. Adult NSCs display high levels of H3K27me3 and require the accumulation of H3K4me3 and expression of DNMT1, HDAC3, and CDKN1C for the faithful generation of OB inhibitory neurons. BAF, Brahma‐associated factor; Cdkn1c, cyclin‐dependent kinase inhibitor 1c; CHD, chromodomain‐helicase‐DNA‐binding; CTCF, CCCCTC‐binding factor; DNMT, DNA methyltransferase; HDAC, histone deacetylase, NSCs, neural stem cell; PRC, polycomb repressive complex; RGP, radial glial progenitor; TET, ten‐eleven translocation protein.
