Figure 1.
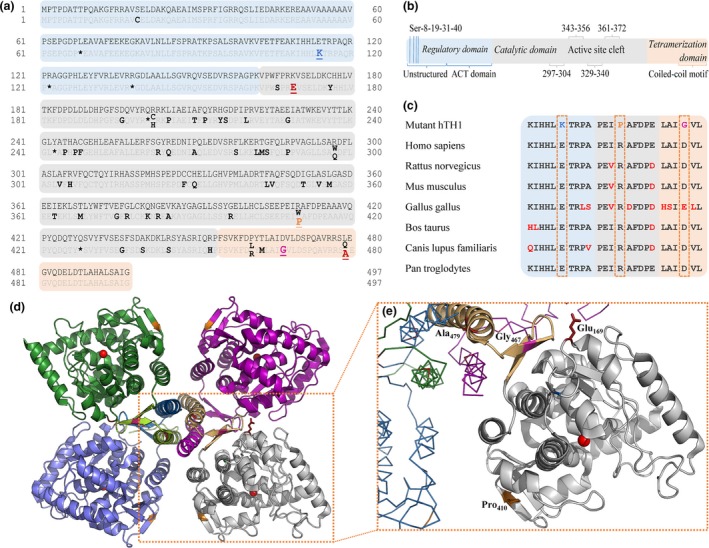
L‐dihydroxyphenylalanine (L‐DOPA)‐responsive dystonia (DRD)‐associated mutations of hTH1. UniProt reference sequence: P07101‐3. (a) All reported disease‐associated missense (bold) and nonsense (asterisk) mutations of the human TH1 gene with varying clinical manifestation (Fossbakk et al. 2014) until January 1st 2018 according to www.biopku.org/pnddb and more recent publications (Yan et al. 2017; Zhang et al. 2017). All mutations investigated in this study are shown underlined (including the experimental K169E/L479A double mutation). (b) A schematic look at the structure of the hTH1 subunit. (c) Amino acid sequence alignment shows that residues Glu‐114, Arg‐410 and Asp‐467 are highly conserved in mammals. (d) Structural overview of TH. The box highlights the catalytic (grey) and oligomerization (light orange) domains of one subunit and their relative positions within the native tetramer (PDB ID: 2XSN). Iron atoms coordinated within the active sites are illustrated with red spheres. Inlet (e) depicts the mutated side chains investigated in this work. Variant E114K is not shown as the N‐terminal 3‐D structure of TH is not yet resolved.
