Abstract
Objective
To investigate distinct features of renal involvement in patients with primary Sjögren’s syndrome (pSS) and to identify potential factors associated with renal involvement.
Methods
Four hundred and thrity‐four pSS patients from the Rheumatology Department of the First Affiliated Hospital of Wenzhou Medical University from 2013 to 2017 were included in a cross‐sectional study. Patients with renal involvement were compared with their age‐ and gender‐matched controls (pSS without renal involvement). Demographic, clinical, histological, nephritic, immunological features of renal involvement in pSS were systematically analyzed. Possible factors related to renal involvement were identified using multivariate logistic regression analyses.
Results
One hundred and ninety‐two pSS patients (88.48%) with renal involvement were women with mean age of nearly 58 years and mean disease duration of above 4 years. Clinical manifestation, serologic and immunological features and renal biopsy class of the pSS patients with renal involvement were presented. By multivariate analyses, xerophthalmia, histological positivity for lower salivary gland biopsy (LSGB), anti‐SSA/Ro52‐positive, reduced complement 3 (C3) levels, hypoalbuminemia and anemia retained significant association with renal involvement in pSS (all P < 0.05).
Conclusion
In addition to LSGB pattern, anti‐SSA/Ro52‐positivity, reduced C3 levels, hypoalbuminemia and anemia, also indicate significant association with renal involvement in pSS. Therefore, early vigilance is required for patients with these clinical manifestations.
Keywords: cross‐sectional studies, multivariate analyses, potential relevant factors, renal involvement, Sjögren’s syndrome
1. INTRODUCTION
Primary Sjögren's syndrome (pSS) is a chronic inflammatory autoimmune connective tissue disorder affecting primarily the lacrimal and salivary glands, resulting in xerophthalmia and xerostomia, which occurs in more than 90% of the patients.1 Sjögren’s syndrome can be primary or secondary to other autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and systemic sclerosis,2 which are all termed as secondary SS (sSS).
Besides exocrine glands involvement, extraglandular manifestations are widely variable, such as arthralgia, myalgia, fatigue and purpura. Even vital organs can be impacted, including interstitial pneumonia, pericarditis, pulmonary hypertension nephritis and neuropathy.3 Although the etiology and pathogenesis of pSS are still obscure, the proliferation of B‐lymphocytes in exocrine glands and extra‐glandular epithelia have been confirmed.4 Therefore, more and more concern has been drawn upon the development of lymphoproliferative disorders.5 Compared to other systemic complications, renal involvement is less frequent with prevalence ranging from 2% to 67% due to different definitions of kidney involvement.6, 7, 8 pSS is reported to precede renal manifestations by a median of 5.5 years in a renal biopsy study of 25 patients.9 Renal involvement in pSS significantly manifests with tubulointerstitial nephritis (TIN) as a result of epithelial disease with significant lymphocytic infiltration which appears to be mainly CD4+ T cells.10
Clinical features of renal involvement in pSS have varied widely,11 and the relationship between the associated factors and renal involvement is seldom investigated. Therefore, we conducted the present study to characterize the demographic, clinical, histological and immunological features of patients with renal involvement in pSS. Furthermore, we investigated the clinical feature and laboratory index which are potentially associated with renal involvement in pSS to facilitate a comprehensive disease evaluation and prevention for pSS patients.
2. MATERIALS AND METHODS
2.1. Study population
We enrolled a total of 434 patients who fulfilled the 2002 classification criteria of the American‐European Consensus Group12 for pSS from the Rheumatology Department of the First Affiliated Hospital of Wenzhou Medical University between 1 January, 2013 and 30 June, 2017 in our study. Within the study cohort, 217 pSS patients were accompanied with renal involvement and another 217 age‐ and gender‐matched pSS without renal involvement were included as controls. The diagnosis of renal involvement was primarily determined by a rheumatologist and confirmed by a nephrologist. To avoid potential interference from other secondary nephropathy, those with suspicious cause of renal lesions from diabetes and hypertension were excluded beforehand. The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University; research design of this study accorded with the current National Health and Family Planning Commission of China ethical standards. In addition, we received written informed consent from all patients prior to enrollment in the study.
2.2. Evaluation of exocrine gland involvement
A detailed history was reviewed and physical examination was taken by the rheumatologists before the pSS patient was recruited into the study. Xerophthalmia was confirmed by ophthalmologists by Schirmer test,13 breakup time of tear film (BUT)14 and cornea fluorescent pigmentation (FL).15 Xerostomia was diagnosed by clinical complaint and whole unstimulated salivary flow (WUSF) test. Histopathological results of lower salivary gland biopsy (LSGB) in these patients were confirmed by the experienced pathologists, and a lymphocytic focus score of ≥1 with more than 50 lymphocytes per 4 mm2 was regarded as positive.16
2.3. Assessment of extraglandular involvement
The systemic condition of patients was evaluated by a thorough review of medical history, physical examination and a series of assessments. We detected nephropathy by altered urine analysis more than twice in the same patient (including proteinuria, microscopic hematuria [≥3 red blood cells per high‐power field in spun urine sediment], abnormal urine pH value and hypokalemia) and serological analysis of hypokalemia and a persistently reduced creatinine clearance.17, 18, 19 Type‐B ultrasonic of the kidney was performed in each patient to assess renal function preliminarily and computed tomography (CT) of the nephridium was used in those with significantly impaired renal function. The kidney puncture was performed in a part of these patients with persistent abnormal urine examination. Immunological tests were performed by using commercial techniques, including antinuclear antibodies (ANA), anti‐Ro antibodies, anti‐SSA antibodies, anti‐La/SSB antibodies using immunoblotting, and rheumatoid factor, complement component 3 (C3) or C4, immunoglobulin g (IgG), IgA, and IgM levels by enzyme‐linked immunosorbent assay (ELISA). Disease manifestations were scored with European League against Rheumatism Sjögren’s syndrome patient reported index and a cumulative European League Against Rheumatism Sjögren’s syndrome disease activity index (ESSDAI) summing the scores achieved per organ domain as previously reported.20
2.4. Data collection and statistical analysis
All data were collected, including demographics, clinical assessment, pathologic findings, and serologic results. We expressed continuous data which fit normal distribution as mean ± standard deviation. Paired comparison of these data was performed using the t test. Continuous data of non‐normal distribution are presented as median with quartiles. Nonparametric Wilcoxon signed‐rank test was performed for paired comparison. Categorical variables were expressed as counts and percentages and comparisons were operated by Fisher’s exact tests. Based on biological plausibility and literature review, univariate analyses were used in our study to examine correlations between multiple factors with renal involvement in pSS. Those variables with positive results in univariate analysis were then analyzed by multivariate regression. Statistical significance was set at P < 0.05. All analyses were conducted using R v3.3.4 statistical software package.21
3. RESULT
3.1. Characteristics of the study cohort and comparisons between pSS patients with and without renal involvement
Demographic, clinical, histological and immunological data were collected from the pSS patients with renal involvement and their age‐ and gender‐matched controls. Comparisons between the two subgroups are presented in Table 1. Each subgroup included 192 (88.48%) women and 25 (11.52%) men. The mean age of hospital presentation was nearly 58 years and mean disease duration on presentation was above 4 years in the cohort, with no differences between the two subgroups. Two subgroups presented similar proportions of diabetes mellitus, hypertension, hyperlipemia and interstitial lung disease (P > 0.05). Although the renal involvement subgroup had lower xerophthalmia prevalence, there was no difference in xerostomia incidence. General symptoms like fever, fatigue and myalgia both involved the two subgroups and had no discrepancy; however, less arthralgia was seen in renal‐involved pSS patients. Of note, the renal‐involved subgroup demonstrated higher histological positivity of LSGB (73.27% vs 63.13%). Regarding the autoantibody profiles, there was no significant difference in ANA‐positive (ANA > 1:320), anti‐SSA/Ro60‐positive and anti‐La/SSB‐positive prevalence between the two subgroups, but anti‐SSA/Ro52‐positive incidence was much higher in those without renal involvement. Also, RF positivity is more often in this subgroup. Nevertheless, elevated erythrocyte sedimentation rate (ESR) and ESSDAI levels and reduced hemoglobin were found in the renal‐involved patients.
Table 1.
Characteristics of the study cohort and comparisons between patients with and without renal involvement in primary Sjögren’s syndrome
| Characteristic | Without renal involvement (N = 217) | With renal involvement (N = 217) | P value |
|---|---|---|---|
| Age, y | 57.31 ± 9.69 | 58.41 ± 15.59 | 0.375 |
| Sex, female (%) | 192 (88.48) | 192 (88.48) | 1.000 |
| Disease duration, mo | 24(6, 72) | 24(5, 60) | 0.9616 |
| Diabetes mellitus, n (%) | 19 (8.76) | 29 (13.36) | 0.126 |
| Hypertension, n (%) | 54 (24.88) | 63 (29.03) | 0.330 |
| Interstitial lung diseases, n (%) | 30 (13.82) | 35 (16.83) | 0.390 |
| Hyperlipemia, n (%) | 113 (52.07) | 100 (46.08) | 0.212 |
| Xerostomia (%) | 119 (59.20) | 118 (58.71) | 0.598 |
| Xerophthalmia (%) | 94 (46.53) | 62 (30.85)* | 0.001 |
| Fatigue, n (%) | 29 (13.43) | 32 (14.75) | 0.693 |
| Fever, n (%) | 36 (16.67) | 43 (19.82 | 0.396 |
| Myalgia, n (%) | 8 (3.86) | 6 (2.87) | 0.574 |
| Arthralgia, n (%) | 67 (32.21) | 51 (24.29) | 0.072 |
| LSGB, lymphocytic focus ≥1, n (%) | 137 (63.13) | 159 (73.27)* | 0.023 |
| ANA‐positive (ANA > 1:320), n (%) | 205 (97.62) | 205 (94.47) | 0.096 |
| Anti‐SSA/Ro60‐positive, n (%) | 152 (72.38) | 154 (71.30) | 0.804 |
| Anti‐SSA/Ro52‐positive, n (%) | 135 (64.29) | 106 (49.07)* | 0.002 |
| Anti‐La/SSB‐positive, n (%) | 89 (42.38) | 102 (47.22) | 0.315 |
| Low C3 levels (<0.9 g/L), n (%) | 95 (47.50) | 120 (57.14) | 0.051 |
| Low C4 levels (<0.1 g/L), n (%) | 6 (3.12) | 12 (5.71) | 0.210 |
| Hypergammaglobulinemia (>16 g/L), n (%) | 122 (57.28) | 114 (53.02) | 0.376 |
| RF‐positive, n (%) | 96 (53.04) | 70 (42.17)* | 0.043 |
| ESR, mm/h | 30.67 ± 21.03 | 40.67 ± 25.13* | <0.001 |
| CRP, mg/dL | 2.55 (1.38, 5.98) | 2.29 (1.51, 8.45) | 0.434 |
| Hemoglobin, g/L | 118 (110, 129) | 109 (99, 124)* | <0.001 |
| ESSDAI | 5 (3, 7) | 7 (5, 9)* | <0.001 |
| ESSPRI | 2.33 (1.66, 3.33) | 2.67 (1.66, 3.33) | 0.791 |
ANA, antinuclear antibodies; C3, complement 3; C4, complement 4; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; ESSDAI, European League Against Rheumatism Sjögren’s syndrome disease activity index; ESSPRI, European League Against Rheumatism Sjögren’s syndrome patient reported index; LSGB, labial salivary gland biopsy; RF, rheumatoid factor.
Data are presented as mean ± standard deviation or as n (%).
Significantly different from corresponding values at P < 0.05.
3.2. Renal manifestations in pSS
Renal manifestations and proportions are presented in Table 2. Almost half of the renal‐involved pSS patients had mild and medium proteinuria (<2.0 g/L). Hematuria (29.5%) and hyposthenuria (17.5%) were also common when tested by routine urine analysis. The serum levels of albumin, creatinine and creatinine clearance rate were 35.13 g/L, 107.04 µmol/L and 62.88 mL/min, demonstrating impairment of renal function in this subgroup. Average urine pH was 6.37% and 31.3% renal‐involved pSS patients had hypokalemia, but only 4.15% had hyperuricemia. In total, there were 64 patients (29.49%) with renal tubular acidosis (RTA) and 60 of them were Type I (distal RTA) and four were Type II (proximal RTA/Fanconi syndrome).
Table 2.
Renal manifestations in primary Sjögren’s syndrome
| Renal characteristics | Case number (%) |
|---|---|
| Proteinuria, n (%) | 116 (53.5) |
| Hematuria, n (%) | 64 (29.5) |
| Hyposthenuria, <1.010, n (%) | 38 (17.5) |
| Albumin, g/L | 35.13 ± 6.55 |
| SCR, µmol/L | 107.04 ± 96.06 |
| CCR, mL/min | 62.88 ± 33.28 |
| Urine pH >6.0, n (%) | 174 (80.2) |
| Hypokalemia, <3.5 mmol/L, n (%) | 68 (31.3) |
| Hyperuricemia, >420 mmol/L, n (%) | 9 (4.15) |
CCR, creatinine clearance rate; SCR, serum creatinine.
Data are presented as mean ± standard deviation or as n (%).
Thirty patients with positive findings by urine analysis in the subgroup received renal biopsy after informed consent. As is shown in Table 3 and Figure 1, besides nine patients suffered from chronic or acute tubulointerstitial nephritis, 25 of the 30 patients had different types of glomerular lesions, including minimal‐change nephropathy, IgA nephropathy, mesangial proliferative glomerulonephritis, focal segmental glomerulosclerosis, diffuse proliferative glomerulonephritis and membranous nephropathy. Some of them shared more than one pathological feature. Membranous nephropathy was the most common pathological type of glomerular lesion which impacted half of the 30 patients, and six of them were atypical membranous nephropathy.
Table 3.
Histological manifestations of renal involvement in primary Sjögren’s syndrome
| Glomerular lesion manifestations | Tubular manifestations | Case number |
|---|---|---|
| Minimally invasive glomerulopathy | — | 2 |
| Immunoglobulin A nephropathy, mesangial proliferative glomerulonephritis with focal segmental glomerulosclerosis | — | 4 |
| Mesangial proliferative glomerulonephritis with focal segmental glomerulosclerosis | Chronic tubulointerstitial nephritis | 1 |
| Focal segmental glomerulosclerosis | — | 1 |
| — | Chronic tubulointerstitial nephritis | 5 |
| Diffuse proliferative glomerulonephritis | — | 1 |
| Diffuse proliferative glomerulonephritis | Chronic tubulointerstitial nephritis | 1 |
| Membranous nephropathy | Acute tubulointerstitial nephritis | 2 |
| Membranous nephropathy | — | 13 |
Figure 1.
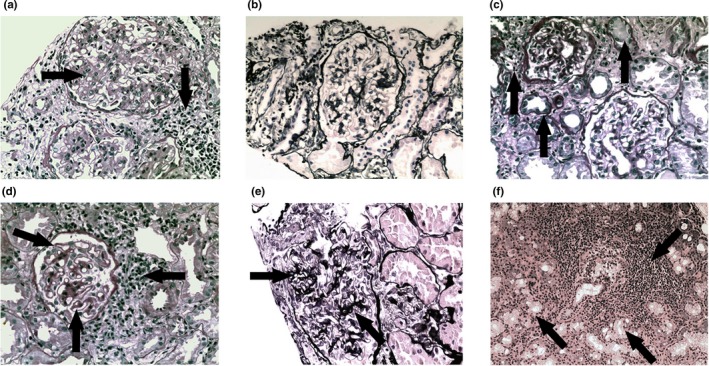
Histologic evaluation of PASM‐stained renal biopsy specimens and H&E‐stained salivary gland biopsy specimens of primary Sjögren’s syndrome (pSS) patients with renal involvement. (A) pSS‐IgAN, MsPGN with FSGS, showing diffuse mesangial hyperplasia with focal interstitial lymphocytic infiltration (arrows) (PASM, 400×); (B) pSS‐cTIN with MsPGN and FSGS, showing diffuse interstitial lymphocytic and plasma infiltration with mild glomerular mesangial hyperplasia (PASM, 400×); (C) pSS‐cTIN, showing multifocal interstitial lymphocytic infiltration with fibrillation, tubular atrophy and protein cast (arrows; PASM, 400×); (D) pSS‐MN with aTIN, showing irregular thickenings out of the glomerular basement membrane as “spikes” with multifocal interstitial lymphocytic and plasma infiltration (arrows; PASM, 400×); (E) pSS‐MN, showing irregular thickenings out of the glomerular basement membrane as “spikes” and “chains” (arrows; PASM, 400×); (F) biopsy specimens from pSS patients with renal involvement showing significant perivascular and periductal lymphocytic infiltration with massive glandular atrophy (arrows; H&E, 200×). aTIN, acute tubulointerstitial nephritis; cTIN, clinical tubulointerstitial nephritis; FSGS, focal segmental glomerulosclerosis; H&E, hematoxylin and eosin‐stained; IgAN, IgA nephropathy; MN, membranous nephropathy; MsPGN, mesangial proliferative glomerulonephritis; PASM, periodic acid‐silver methenamine‐stained.
3.3. Characteristics of high titer ANA‐positive patients in pSS with renal involvement
As shown in Table 4, high titer ANA (1:320) was more common (58.99%) in the renal‐involved pSS. Also, the ANA‐positive subgroup had a higher female proportion, more cutaneous domain involvement and positive histological findings of LSGB. Unsurprisingly, patients with ANA positivity presented higher anti‐SSA/SSB and RF positive rates and increased IgA levels. However, the ANA negative subgroup manifested more severe renal impairment with much lower creatinine clearance rate (CCR) levels, but without significant difference in ESSDAI with the positive subgroup.
Table 4.
Analysis of multiple features of primary Sjögren’s syndrome patients with renal involvement according to ANA positivity
| Characteristic | ANA negative (n = 89) | ANA positive (n = 128) | P value |
|---|---|---|---|
| Age, y | 59.64 ± 15.43 | 57.56 ± 15.71 | 0.333 |
| Sex, female (%) | 73 (82.02) | 119 (92.97)* | 0.013 |
| Disease duration, mo | 24 (4, 60) | 24 (5, 81) | 0.638 |
| Cutaneous domain involvement, n (%) | 6 (7.69) | 22 (18.64)* | 0.032 |
| LSGB, lymphocytic focus ≥1, n (%) | 58 (65.17) | 101 (78.91)* | 0.024 |
| Anti‐SSA/Ro60‐positive, n (%) | 54 (60.67) | 100 (78.74)* | 0.004 |
| Anti‐La/SSB‐positive, n (%) | 32 (35.96) | 70 (55.12)* | 0.005 |
| Anti‐SSA/Ro52‐positive, n (%) | 35 (39.33) | 71 (55.91)* | 0.016 |
| Low C3 levels, <0.9 g/L, n (%) | 49 (56.32) | 71 (57.72) | 0.840 |
| Low C4 levels, <0.1 g/L, n (%) | 27 (31.03) | 32 (26.02) | 0.425 |
| IgG levels, g/L | 20.11 ± 14.03 | 21.68 ± 10.46 | 0.374 |
| IgA levels, g/L | 3.02 ± 1.71 | 3.78 ± 2.10 * | 0.003 |
| RF‐positive, n (%) | 17 (23.61) | 53 (56.38)* | <0.001 |
| SCR, µmol/L | 82 (67, 115) | 77 (61, 105) | 0.121 |
| CCR, mL/min | 57.07 ± 33.42 | 66.93 ± 32.71* | 0.038 |
| Hemoglobin, g/L | 110.43 ± 20.02 | 110.70 ± 17.82 | 0.916 |
| CRP, mg/dL | 2.92 (1.76, 9.94) | 2.11 (1.36, 7.91) | 0.122 |
| ESR, mm/h | 39.74 ± 26.51 | 41.31 ± 24.22 | 0.673 |
| ESSDAI | 8 (5, 9) | 7 (5, 9) | 0.809 |
| ESSPRI | 2.33 (2, 3) | 2.67 (1.67, 3.33) | 0.793 |
ANA, antinuclear antibodies; C3, complement 3; C4, complement 4; CCR, creatinine clearance rate; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; ESSDAI, European League Against Rheumatism Sjögren’s syndrome disease activity index; ESSPRI, European League Against Rheumatism Sjögren’s syndrome patient reported index; LSGB, labial salivary gland biopsy; RF, rheumatoid factor; SCR, serum creatinine.
Data are presented as mean ± standard deviation or as n (%).
Significantly different from corresponding values at P < 0.05.
3.4. Features of different histological patterns of LSGB in pSS with kidney involvement
As shown in Table 5, positive histological findings (lymphocytic focus score ≥1) were detected in 73.27% of the pSS patient with renal involvement. General manifestation like fatigue was more common in the histologically positive group (P < 0.05). Also, this subgroup had much higher levels of ESSDAI, IgG, lower level of hemoglobin and CCR, and more frequent low C3 levels prevalence (P < 0.05). All these indices suggested much more severe conditions in these patients than the negative subgroup.
Table 5.
Analysis of multiple features of primary Sjögren’s syndrome patients with renal involvement according to histological pattern of LSGB
| Characteristic | LSGB negative (n = 58) | LSGB positive (n = 159) | P value |
|---|---|---|---|
| Age, y | 58.69 ± 16.12 | 58.31 ± 15.44 | 0.878 |
| Disease duration, mo | 24 (3.5, 60) | 24 (5, 60) | 0.833 |
| Fatigue, n (%) | 2 (3.45) | 30 (18.87)* | 0.005 |
| ANA‐positive, ANA >1:320, n (%) | 27 (46.55) | 101 (63.52)* | 0.024 |
| Low C3 levels, <0.9 g/L, n (%) | 24 (42.86) | 96 (62.34)* | 0.012 |
| Low C4 levels, <0.1 g/L, n (%) | 14 (24.56) | 45 (29.41) | 0.487 |
| IgG levels, g/L | 16.08 ± 7.16 | 22.86 ± 12.97* | <0.001 |
| SCR, µmol/L | 73 (57, 89.25) | 82 (67, 115) | 0.005 |
| CCR, mL/min | 70.52 ± 35.66 | 60.02 ± 32.00* | 0.046 |
| Hemoglobin, g/L | 115.53 ± 18.66 | 108.79 ± 18.46* | 0.019 |
| Albumin, g/L | 32.61 ± 7.56 | 36.05 ± 5.91* | 0.002 |
| CRP, mg/dL | 3.62 (1.84, 9.36) | 2.23 (1.39, 8.35) | 0.070 |
| ESR, mm/h | 40.30 ± 26.07 | 40.81 ± 24.84 | 0.901 |
| ESSDAI | 6 (4.25, 8.75) | 8 (6, 9)* | 0.016 |
| ESSPRI | 2.67 (1.33, 3) | 2.67 (2, 3.33) | 0.747 |
ANA, antinuclear antibodies; C3, complement 3; C4, complement 4; CCR, creatinine clearance rate; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; ESSDAI, European League Against Rheumatism Sjögren’s syndrome disease activity index; ESSPRI, European League Against Rheumatism Sjögren’s syndrome patient reported index; LSGB, labial salivary gland biopsy; RF, rheumatoid factor; SCR, serum creatinine.
Data are presented as mean ± standard deviation or as n (%).
Significantly different from corresponding values at P < 0.05.
3.5. Specific factors associated with renal involvement in pSS
The associations between the various factors in pSS patients with renal involvement were first assessed using univariate analyses (Table 6). Several factors were significantly associated with renal involvement, including xerophthalmia, positive histological findings of LSGB, anti‐SSA/Ro52 positivity, IgG level, reduced C3 levels (C3 < 0.80 g/L), ESR level, hypoalbuminemia and anemia (all P < 0.05).
Table 6.
Univariate analyses of factors associated with renal involvement in primary Sjögren’s syndrome
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Xerophthalmia, n (%) | 0.51 | 0.34‐0.77 | 0.001 |
| LSGB, lymphocytic focus ≥1, n (%) | 1.60 | 1.06‐2.41 | 0.024 |
| Anti‐SSA/Ro52‐positive, n (%) | 0.54 | 0.36‐0.79 | 0.002 |
| Hypergammaglobulinemia, >16 g/L, n (%) | 1.03 | 1.00‐1.05 | 0.016 |
| Low C3 levels, <0.9 g/L, n (%) | 1.97 | 1.30‐3.01 | 0.024 |
| Elevated ESR levels, >20 mm/h, n (%) | 1.02 | 1.01‐1.03 | <0.001 |
| Hypoalbumin, <35 g/L, n (%) | 1.90 | 1.30‐2.96 | 0.003 |
| Anemia, <110 g/L), n (%) | 3.20 | 2.13‐4.85 | <0.001 |
ESR, erythrocyte sedimentation rate; LSGB, labial salivary gland biopsy.
To avoid interference from potentially related factors, these factors associated with renal involvement were further analyzed by multivariate analyses (Table 7). In the multivariate analysis, xerophthalmia, histological positivity of LSGB, anti‐SSA/Ro52 positivity, reduced C3 levels, hypoalbuminemia and anemia retained significant association with renal involvement in pSS (all P < 0.05). Interestingly, xerophthalmia and anti‐SSA/Ro52 positivity were negatively associated with renal involvement in pSS.
Table 7.
Multivariable analysis of factors associated with renal involvement in primary Sjögren’s syndrome
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Xerophthalmia, n (%) | 0.52 | 0.33‐0.82 | 0.005 |
| LSGB, Lymphocytic focus ≥1, n (%) | 1.99 | 1.23‐3.26 | 0.006 |
| Low C3 levels, <0.9 g/L, n (%) | 2.09 | 1.29‐3.42 | 0.003 |
| Anti‐SSA/Ro52‐positive, n (%) | 0.41 | 0.26‐0.65 | 0.002 |
| Hypoalbumin, <35 g/L, n (%) | 1.93 | 1.18‐3.19 | 0.003 |
| Anemia, <110 g/L, n (%) | 2.59 | 1.62‐4.19 | <0.001 |
LSGB, labial salivary gland biopsy.
4. DISCUSSION
To date our study represents one of the largest cross‐sectional study cohorts of Chinese pSS patients with renal involvement. Till now, few studies have specially investigated the renal features and potential factors associated with renal involvement in pSS patients. Our cohort has a higher prevalence of renal involvement in women but with similar onset ages and disease duration compared with a previous study.9 However, there was lower anti‐SSA/RO‐52 positivity compared to previous reports22, 23 (49.07% vs 72.2%~77.1%). Also the pulmonary involvement was varied from these two studies. Whether this difference is due to genetic, territorial causes or definition of renal involvement warrants further investigation.
Within our cohort, the renal‐involved pSS patients displayed a distinctive clinical profile with higher disease activity (ESSDAI), more LSGB positivity and elevated inflammation levels (as reflected by ESR), but lower xerophthalmia prevalence compared with their age‐ and gender‐matched nonrenal‐involved controls.
Antinuclear antibodies positivity was defined in our cohort as a 1:320 titer or higher to exclude low titer ANA positivity as it’s not so specific especially in older people or those with other chronic diseases. Further, it is suggested that ANA 1:320 positivity and RF (especially IgA type of RF) are both related to the presence of anti‐SSA antibodies, and ANA 1:640 positivity and blood B‐cell subset profiling have good diagnostic properties for pSS.24 Therefore, high titer ANA positivity is much more significant in pSS. In our study, high titer ANA positivity was more common in renal‐involved pSS patients with a higher proportion of cutaneous involvement, although the total incident rate of cutaneous involvement in pSS patients was not as high as reported in previous studies.25, 26 Moreover, the ANA positive subgroup indicated elevated levels of IgA and RF, and were consistent with higher LSGB positivity just as previously reported.27 Of note, in our study, more severe renal impairment was observed in the ANA negative subgroup. The reason is still not clear, so more attention should be given to those without significant immunological antibody.
Lymphocytic infiltration in the labial gland with a focus score of ≥1 per 4 mm2 is the phenotypic feature of pSS.28 In the histopathologically positive subgroup, a mean ESSDAI of 7.67 was observed, indicating medium disease activity. Importantly, a majority of renal‐involved pSS patients had positive histopathology, in accordance with higher proportion of ESSDAI index, anemia, elevated level of IgG and low level of C3, indicating a close correlation between histological patterns and severity and activity of the disease in those patients, which was also found in whole pSS cohorts in previous studies.29, 30 Higher SGUS (salivary gland ultrasonography) score (3 and 4) has already been found to be was associated with elevated IgG (>1600 mg/dL) and ESSDAI (≥5) levels, further suggesting the relationship between disease severity and dysfunction of the salivary gland.31
Chronic tubulointerstitial nephritis (TIN) is the most common renal manifestation of SS,32, 33 which mainly comprise three major forms of RTA: Types I and IV distal RTA and proximal RTA (Type II). Despite an incomplete variant of the disease and retaining normal serum bicarbonate levels, all of these types have an alkaline urine pH34; therefore we used urine pH as a requisite, although this was not sufficient feature in the present study to reflect tubulointerstitial involvement. It was revealed that 80.2% of the renal‐involved cohort got urine pH >6.0, implying the high prevalence of TIN in pSS. However, glomerular disease is far from rare in our cohort. Renal biopsy revealed diverse pathological findings, including membranoproliferative glomerulonephritis (MPGN), membranous nephropathy (MN), minimal change disease, IgA nephropathy, and so on. The etiology and pathogenesis of the glomerular disease in pSS are unclear but may be related to the deposition of circulating immune complexes, even underlying cryoglobulinemia with a late sequela and a less favorable prognosis.35, 36 As proteinuria is much more likely to be indicative of renal biopsy than the typical clinical characteristic of TIN, more glomerular disease, especially MN, was found by renal biopsy. The prognosis of patients with predominantly glomerular involvement was found to be worse with lower survival rates and a higher incidence of lymphoma,37, 38 so renal biopsy is still suggested for those with suspicious glomerular disease.
Renal involvement is hard to foresee but directly influences the prognosis of pSS patients. In our study, xerophthalmia, positivity of labial gland biopsy, anti‐SSA/Ro52 positivity, reduced C3 levels, hypoalbuminemia and anemia had been confirmed to be significantly associated with renal involvement in pSS by univariate and multivariate analyses. Of note, xerophthalmia manifested negative association with renal involvement, which was also found in a previous study.23 Also, anti‐SSA/Ro52 positivity was also found negatively associated with renal involvement in our study. The reasons are still unclear, and further studies are required. It’s presumed that uncontrolled activation of the complement pathway leads to predominantly glomerular deposition of C3, which is called C3 glomerulopathy.39 Based on that, we believe reduced C3 levels, as a result of overconsuming, may be highly associated with renal involvement. Anemia in pSS can be caused by autoimmune hemolysis, iron deficiency, chronic diseases, like kidney disease, and so on. It’s suggested to measure hemoglobin in patients with impaired renal function to identify those with a higher risk of having or developing anemia, and to assess renal function using a simple and reliable formula based on their mutual relationship, which accords with our study.40 Furthermore, anemia and hypoalbuminemia were associated with increased risk of morbidity and mortality in end‐stage renal disease,41 which seems to fit in with our findings.
Our study may have several limitations. First, limited pSS with renal involvement patients had renal biopsy which could create selection bias, and might lead to a high prevalence of the glomerulonephritis pattern. Also, as a cross‐section study, our study is only limited to making correlation analysis rather than causality analysis. For example, it’s difficult to determine whether anemia and hypoproteinemia are the causes or results of renal involvement in pSS. Future multi‐center prospective studies are needed to confirm our findings.
In conclusion, chronic tubulointerstitial inflammation is the most typical renal manifestation in pSS, while glomerular involvement is also frequent. Positive labial gland biopsy is highly associated with renal lesions. Other factors, including anti‐SSA/Ro52 positivity, reduced C3 levels, hypoalbuminemia and anemia, also indicate a significant association with renal involvement in pSS. Therefore, early vigilance is required for patients with these clinical manifestations.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
XZ and XW conducted the study conception and design; JL, SX and HZ acquired, analyzed and interpreted the data. JL, XH and YL composed the article.
ACKNOWLEDGEMENTS
We thank the patients for participating in this study. We also thank Professor Jianmin Li for providing expert advice on salivary gland and renal biopsy pathology.
Luo J, Xu S, Lv Y, et al. Clinical features and potential relevant factors of renal involvement in primary Sjögren’s syndrome. Int J Rheum Dis. 2019;22:182–190. 10.1111/1756-185X.13429
REFERENCES
- 1. Ramos‐Casals M, Solans R, Rosas J, et al. Primary Sjogren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008;87(4):210‐219. [DOI] [PubMed] [Google Scholar]
- 2. Theander E, Jacobsson LT. Relationship of Sjögren's syndrome to other connective tissue and autoimmune disorders. Rheum Dis Clin North Am. 2008;34(4):935‐947. [DOI] [PubMed] [Google Scholar]
- 3. Ramos‐Casals M, Brito‐Zeron P, Siso‐Almirall A, Bosch X. Primary Sjogren syndrome. BMJ. 2012;344:e3821. [DOI] [PubMed] [Google Scholar]
- 4. Sjögren H. Zur kenntnis der keratoconjunctivitis sicca II. Acta Ophthalmol. 2010;13(1‐2):1‐39. [DOI] [PubMed] [Google Scholar]
- 5. Fox RI, Liu AY. Sjogren's syndrome in dermatology. Clin Dermatol. 2006;24(5):393‐413. [DOI] [PubMed] [Google Scholar]
- 6. Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36(3):340‐347. [DOI] [PubMed] [Google Scholar]
- 7. Pertovaara M, Korpela M, Pasternack A. Factors predictive of renal involvement in patients with primary Sjogren's syndrome. Clin Nephrol. 2001;56(1):10‐18. [PubMed] [Google Scholar]
- 8. Bossini N, Savoldi S, Franceschini F, et al. Clinical and morphological features of kidney involvement in primary Sjogren's syndrome. Nephrol Dial Transplant. 2001;16(12):2328‐2336. [DOI] [PubMed] [Google Scholar]
- 9. Kidder D, Rutherford E, Kipgen D, Fleming S, Geddes C, Stewart GA. Kidney biopsy findings in primary Sjögren syndrome. Nephrol Dial Transplant. 2015;30(8):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 10. Evans RD, Laing CM, Ciurtin C, Walsh SB. Tubulointerstitial nephritis in primary Sjogren syndrome: clinical manifestations and response to treatment. BMC Musculoskelet Disord. 2016;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bossini N, Savoldi S, Franceschini F, et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dial Transplant. 2001;16(12):2328. [DOI] [PubMed] [Google Scholar]
- 12. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis. 2002;61(6):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261‐1273. [DOI] [PubMed] [Google Scholar]
- 14. Goren MB, Goren SB. Diagnostic tests in patients with symptoms of keratoconjunctivitis sicca. Am J Ophthalmol. 1988;106(5):570‐574. [DOI] [PubMed] [Google Scholar]
- 15. Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren's Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guellec D, Cornec D, Joussejoulin S, et al. Diagnostic value of labial minor salivary gland biopsy for Sjogren's syndrome: a systematic review. Autoimmun Rev. 2013;12(3):416‐420. [DOI] [PubMed] [Google Scholar]
- 17. Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy‐documented renal involvement in primary Sjögren syndrome. Medicine. 2000;79(4):241‐249. [DOI] [PubMed] [Google Scholar]
- 18. Poux JM, Peyronnet P, Le MY, Favereau JP, Charmes JP, Lerouxrobert C. Hypokalemic quadriplegia and respiratory arrest revealing primary Sjogren's syndrome. Clin Nephrol. 1992;37(4):189‐191. [PubMed] [Google Scholar]
- 19. Cohen EP, Bastani B, Cohen MR, Kolner S, Hemken P, Gluck SL. Absence of H(+)‐ATPase in cortical collecting tubules of a patient with Sjogren's syndrome and distal renal tubular acidosis. J Am Soc Nephrol. 1992;3(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 20. Risselada AP, Kruize AA, Bijlsma JW. Clinical applicability of the EULAR Sjogren's syndrome disease activity index: a cumulative ESSDAI score adds in decreatinineibing disease severity. Ann Rheum Dis. 2012;71(4):631. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org/. Accessed May 6, 2018. [Google Scholar]
- 22. Zhao Y, Li Y, Wang L, et al. Primary Sjögren syndrome in Han Chinese: clinical and immunological characteristics of 483 patients. Medicine. 2015;94(16):e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang HX, Wang J, Wen YB, et al. Renal involvement in primary Sjögren's syndrome: a retrospective study of 103 biopsy‐proven cases from a single center in China. Int J Rheum Dis. 2017;21(1):223‐229. [DOI] [PubMed] [Google Scholar]
- 24. Cornec D, Saraux A, Pers JO, et al. Diagnostic accuracy of blood B‐cell subset profiling and autoimmunity markers in Sjögren’s syndrome. Arthritis Res Ther. 2014;16(1):R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Connolly MK. Sjogern's syndrome. Semin Cutan Med Surg. 2001;20(1):46. [DOI] [PubMed] [Google Scholar]
- 26. Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data‐driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012;64(4):475‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos‐Casals M, Font J, Garcia‐Carrasco M, et al. Primary Sjögren syndrome: hematologic patterns of disease expression. Medicine. 2002;81(4):281‐292. [DOI] [PubMed] [Google Scholar]
- 28. Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren's syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63:2021‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Theander E, Mandl T. Primary Sjögren's syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res (Hoboken). 2014;66(7):1102‐1107. [DOI] [PubMed] [Google Scholar]
- 30. Hammenfors DS, Brun JG, Jonsson R, Jonsson MV. Diagnostic utility of major salivary gland ultrasonography in primary Sjögren's syndrome. Clin Exp Rheumatol. 2015;33(1):56‐62. [PubMed] [Google Scholar]
- 31. Fidelix T, Czapkowski A, Azjen S, Andriolo A, Trevisani V. Salivary gland ultrasonography as a predictor of clinical activity in Sjögren’s syndrome. Plos One. 2017;12(8):e0182287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kidder D, Rutherford E, Kipgen D, Fleming S, Geddes C, Stewart GA. Kidney biopsy findings in primary Sjogren syndrome. Nephrol Dial Transplant. 2015;30(8):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 33. Maripuri S, Grande JP, Osborn TG, et al. Renal involvement in primary Sjogren's syndrome: a clinicopathologic study. Clin J Am Soc Nephrol. 2009;4(9):1423‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy P. Clinical approach to renal tubular acidosis in adult patients. Int J Clin Pract. 2011;65(3):350‐360. [DOI] [PubMed] [Google Scholar]
- 35. Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy‐documented renal involvement in primary Sjogren syndrome. Medicine (Baltimore). 2000;79(4):241‐249. [DOI] [PubMed] [Google Scholar]
- 36. Matignon M, Cacoub P, Colombat M, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore). 2009;88(6):341‐348. [DOI] [PubMed] [Google Scholar]
- 37. Goules AV, Tatouli IP, Moutsopoulos HM, Tzioufas AG. Clinically significant renal involvement in primary Sjögren's syndrome: clinical presentation and outcome. Arthritis Rheum. 2013;65(11):2945‐2953. [DOI] [PubMed] [Google Scholar]
- 38. Francois H, Mariette X. Renal involvement in primary Sjogren syndrome. Nat Rev Nephrol. 2016;12(2):82‐93. [DOI] [PubMed] [Google Scholar]
- 39. Welte T, Arnold F, Kappes J, et al. Treating C3 glomerulopathy with eculizumab. BMC Nephrol. 2018;19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippi G, Franchini M, Salvagno GL, Montagnana M, Targher G, Guidi GC. Determinants of anaemia in the very elderly: a major contribution from impaired renal function? Blood Transfus. 2010;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sit D, Kadiroglu AK, Kayabasi H, Kara IH, Yilmaz Z, Yilmaz ME. The evaluation incidence and risk factors of mortality among patients with end stage renal disease in southeast Turkey. Ren Fail. 2008;30(1):37. [DOI] [PubMed] [Google Scholar]


